Benzene-containing selenium group substituted diheterocyclic compound as well as preparation and application thereof
The technology of a phenylselenyl compound is applied in the application field of preparing monoamine oxidase inhibitors, and can solve the problems of large toxic and side effects, single action effect of monoamine oxidase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
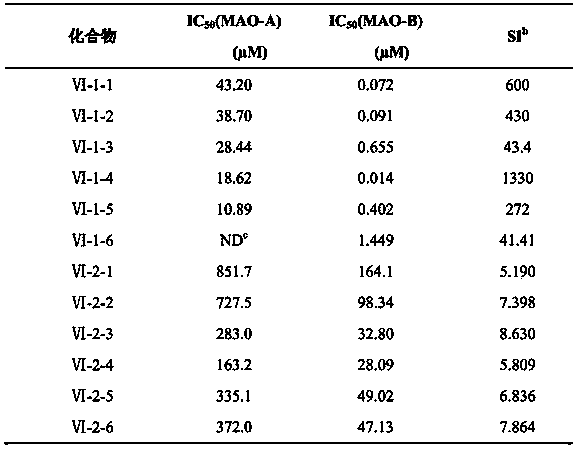
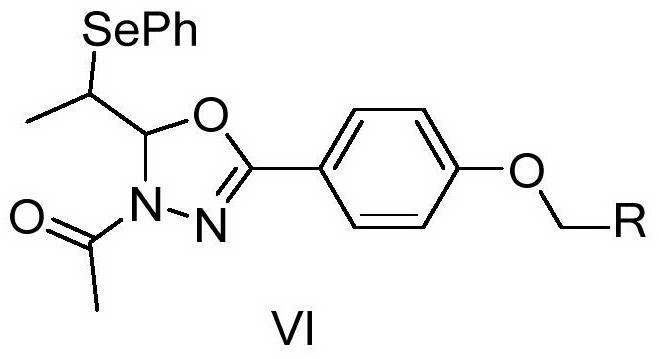
[0074] Example 1 Synthesis of oxadiazole derivatives VI-1-1. containing isoxazole
[0075]
[0076] 1) Add an equivalent amount of α-phenylselenopropionaldehyde (0.213g, 1mmol) and p-hydroxyphenylhydrazine (1mmol, 0.152g) into a round-bottomed flask containing 20ml of ethanol, stir to dissolve them completely, and then add 800μL of acetic acid , Reflux reaction under nitrogen protection for 3h. After TLC detected that the reaction was complete, the ethanol was drained, and 20mL CH 2 Cl 2 , with saturated Na 2 CO 3 solution (3 × 20 mL) washed with aqueous solution, anhydrous Na 2 SO 4 After drying and concentration, the crude product of intermediate product (Ⅲ) containing phenylselenyl group was obtained.
[0077] 2) The obtained product (Ⅲ) (1 mmol) was added to 5 mL of acetic anhydride, heated to reflux for 3 h under the protection of nitrogen, the color of the solution changed to orange and suspended solids were formed. After TLC detection reaction is complete, dra...
Embodiment 2
[0081] Example 2 Synthesis of oxadiazole derivatives VI-1-2. containing isoxazole
[0082]
[0083] 1) operate as embodiment 1 step 1
[0084] 2) operate as embodiment 1 step 2
[0085] 3) operate as embodiment 1 step 3
[0086] 4) Dissolve p-methoxybenzoxime (2mmol, 0.288g) and equivalent NCS (2mmol, 0.268g) in 20mL CH 2 Cl 2 After reacting at room temperature for 3h, compound V (1mmol, 0.388g) was added and triethylamine (2mmol, 0.28mL) was added dropwise to react for 6 hours. After most of the reaction was detected by TLC, subsequent treatment was as in step 4 of Example 1. VI-1-2. was obtained by thin-plate chromatography with a yield of 85%. Product characterization results are as follows:
[0087] 1 H NMR (400MHz, CDCl 3 )δ7.75-7.72(m,4H),7.68-7.64(m,4H),7.30(d,J=3.5Hz,2H),7.12-7.10(m,3H),6.99(d,J=8.8Hz ,1H),6.62(s,1H),5.24(s,2H),3.94-3.62(m,1H),3.85(s,3H),2.31(s,3H),1.34(d,J=7.2Hz, 3H); IRν max (cm -1 ):3120,2976,2920,2820,1665,1607,1580,1562,1504,1455,140...
Embodiment 3
[0088] Example 3 Synthesis of Isoxazole-containing Oxadiazole Derivatives VI-1-3.
[0089]
[0090]1) operate as embodiment 1 step 1
[0091] 2) operate as embodiment 1 step 2
[0092] 3) operate as embodiment 1 step 3
[0093] 4) Dissolve p-chlorobenzoxime (2mmol, 0.313g) and equivalent NCS (2mmol, 0.268g) in 20mL CH 2 Cl 2 After reacting at room temperature for 3h, compound V (1mmol, 0.388g) was added and triethylamine (2mmol, 0.28mL) was added dropwise to react for 6 hours. After most of the reaction was detected by TLC, subsequent treatment was as in step 4 of Example 1. VI-1-3. was obtained by thin-plate chromatography with a yield of 71%. Product characterization results are as follows:
[0094] 1 H NMR (400MHz, CDCl 3 )δ7.68-7.60(m,4H),7.52(d,J=8.5Hz,2H),7.37–7.17(m,7H),6.92(d,J=8.5Hz,1H),6.35(s,1H ), 4.10(dd, J=49.1, 14.1Hz, 1H), 2.47(s, 2H), 2.40(s, 3H), 1.25(d, J=4.6Hz, 3H); IRν max (cm -1 ):3031,2922,2867,1884,1760,1618,1578,1551,1487,1445,1398,1376,120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com