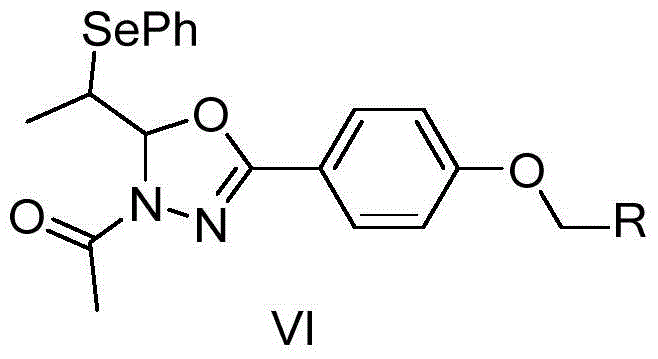
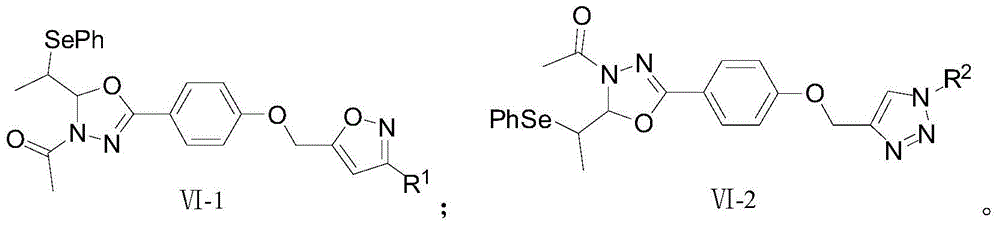
Biheterocyclic compound containing phenylselenyl substitution and its preparation and application
The technology of a phenylselenium compound, which is applied in the application field of preparing monoamine oxidase inhibitors, can solve the problems of high toxicity and side effects, single effect of monoamine oxidase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Synthesis of oxadiazole derivatives VI-1-1. containing isoxazole
[0075]
[0076] 1) Add an equivalent amount of α-phenylselenopropionaldehyde (0.213g, 1mmol) and p-hydroxyphenylhydrazine (1mmol, 0.152g) into a round-bottomed flask containing 20ml of ethanol, stir to dissolve them completely, and then add 800μL of acetic acid , Reflux reaction under nitrogen protection for 3h. After TLC detected that the reaction was complete, the ethanol was drained, and 20mL CH 2 Cl 2 , with saturated Na 2 CO 3 solution (3 × 20 mL) washed with aqueous solution, anhydrous Na 2 SO 4 After drying and concentration, the crude product of intermediate product (Ⅲ) containing phenylselenyl group was obtained.
[0077] 2) The obtained product (Ⅲ) (1 mmol) was added to 5 mL of acetic anhydride, heated to reflux for 3 h under the protection of nitrogen, the color of the solution changed to orange and suspended solids were formed. After TLC detection reaction is complete, dra...
Embodiment 2
[0081] Example 2 Synthesis of oxadiazole derivatives VI-1-2. containing isoxazole
[0082]
[0083] 1) operate as embodiment 1 step 1
[0084] 2) operate as embodiment 1 step 2
[0085] 3) operate as embodiment 1 step 3
[0086] 4) Dissolve p-methoxybenzoxime (2mmol, 0.288g) and equivalent NCS (2mmol, 0.268g) in 20mL CH 2 Cl 2 After reacting at room temperature for 3h, compound V (1mmol, 0.388g) was added and triethylamine (2mmol, 0.28mL) was added dropwise to react for 6 hours. After most of the reaction was detected by TLC, subsequent treatment was as in step 4 of Example 1. VI-1-2. was obtained by thin-plate chromatography with a yield of 85%. Product characterization results are as follows:
[0087] 1 H NMR (400MHz, CDCl 3 )δ7.75-7.72(m,4H),7.68-7.64(m,4H),7.30(d,J=3.5Hz,2H),7.12-7.10(m,3H),6.99(d,J=8.8Hz ,1H),6.62(s,1H),5.24(s,2H),3.94-3.62(m,1H),3.85(s,3H),2.31(s,3H),1.34(d,J=7.2Hz, 3H); IRν max (cm -1 ):3120,2976,2920,2820,1665,1607,1580,1562,1504,1455,140...
Embodiment 3
[0088] Example 3 Synthesis of Isoxazole-containing Oxadiazole Derivatives VI-1-3.
[0089]
[0090] 1) operate as embodiment 1 step 1
[0091] 2) operate as embodiment 1 step 2
[0092] 3) operate as embodiment 1 step 3
[0093] 4) Dissolve p-chlorobenzoxime (2mmol, 0.313g) and equivalent NCS (2mmol, 0.268g) in 20mL CH 2 Cl 2 After reacting at room temperature for 3h, compound V (1mmol, 0.388g) was added and triethylamine (2mmol, 0.28mL) was added dropwise to react for 6 hours. After most of the reaction was detected by TLC, subsequent treatment was as in step 4 of Example 1. VI-1-3. was obtained by thin-plate chromatography with a yield of 71%. Product characterization results are as follows:
[0094] 1 H NMR (400MHz, CDCl 3 )δ7.68-7.60(m,4H),7.52(d,J=8.5Hz,2H),7.37–7.17(m,7H),6.92(d,J=8.5Hz,1H),6.35(s,1H ), 4.10(dd, J=49.1, 14.1Hz, 1H), 2.47(s, 2H), 2.40(s, 3H), 1.25(d, J=4.6Hz, 3H); IRν max (cm -1 ):3031,2922,2867,1884,1760,1618,1578,1551,1487,1445,1398,1376,1206...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com