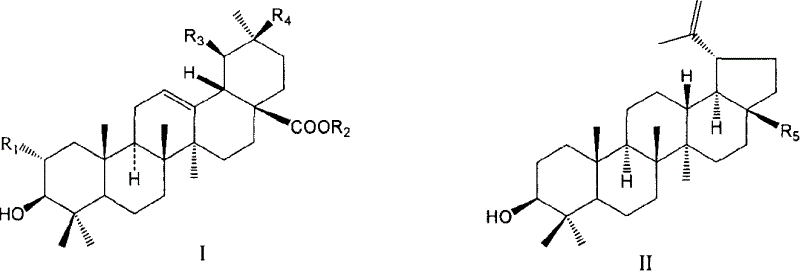
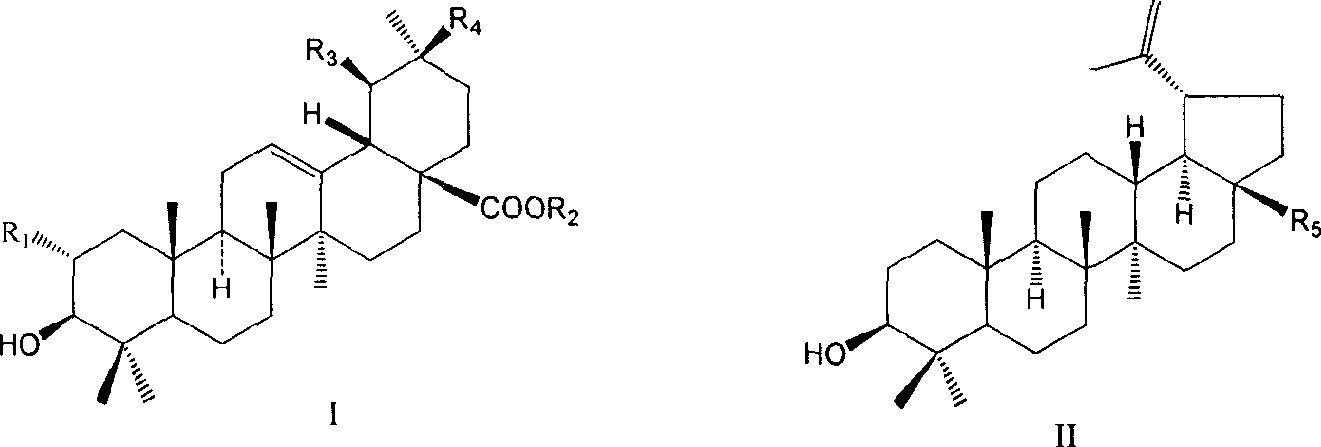
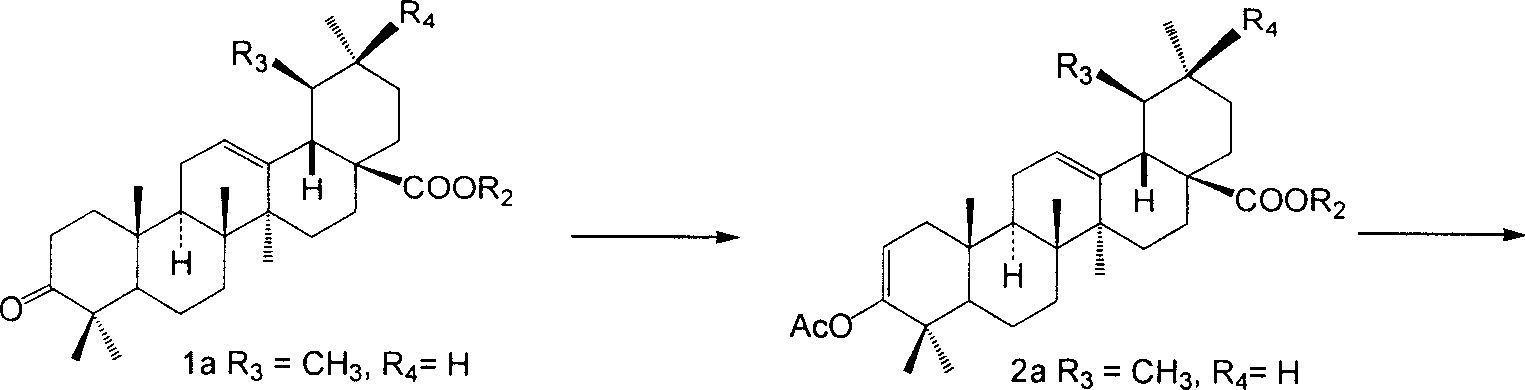
Use of pentacylic triterpene compounds in preparing glycogenic phosphorylase inhibitor
A technology of glycogen phosphorylase and pentacyclic triterpenoids, which is applied in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Preparation of pentacyclic triterpenoids:
[0033] Reagents and test methods: Infrared spectrum was measured with NieoletImpact 410 IR spectrometer, and KBr pellets were used; 1HNMR and 13CNMR were measured with ACF-300(500) BRUK nuclear magnetic resonance; MS was measured with HP1100 mass spectrometer. Ursolic acid (purity about 95%) was purchased from Shaanxi Huike Plant Development Co., Ltd.; oleanolic acid (purity about 97%) was purchased from Chengdu Super Human Phytochemical Development Co., Ltd.; white birch bark was collected from Inner Mongolia; other reagents and solvents All commercially available chemically pure or analytically pure products were used directly without treatment unless otherwise specified.
Embodiment 1
[0034] Embodiment 1: the preparation of maslinic acid and its benzyl ester
[0035] Oleanolic acid (10.0g) was suspended in 100mL of anhydrous DMF, heated at 100°C to dissolve completely, and then added K 2 CO 3 (6.04g) and benzyl chloride (3.0mL). The mixture was heated at 100°C with stirring until the starting material disappeared (ca. 3 hours). After cooling, filter with suction, and wash the solid with DMF 3 times, 15 mL each time. Pour the mother liquor into 500mL of water, shake while pouring to disperse the precipitated solids, let stand until the solids are completely precipitated, collect the solids by suction filtration, and wash them thoroughly with water. After drying, 11.51 g of white crude benzyl oleanate was obtained (crude yield 96%). The crude product can be directly used in the next reaction. 1 HNMR (CDCl 3 , 500MHz): δ0.62, 0.78, 0.88, 0.90, 0.92, 0.98, 1.13(each, 3H, s), 2.91(1H, dd, J=4.4, 13.9Hz, H-18), 3.20(1H, dd , J=4.5, 11.2Hz, H-3α), 5.07 (2H,...
Embodiment 2
[0040] Embodiment 2: the preparation of benzyl ursolic acid, corosolic acid and benzyl ester thereof
[0041] Suspend ursolic acid (10.0g) in 100mL of anhydrous DMF, heat at 100°C to dissolve completely, let stand for a little cooling, then add K 2 CO 3 (6.04g) and benzyl chloride (3.0mL). The mixture was heated at 100°C with stirring until the starting material disappeared (ca. 3 hours). After cooling, filter with suction, and wash the solid with DMF 3 times, 15 mL each time. Pour the mother liquor into 500mL of water, shake while pouring to disperse the precipitated solids, let stand until the solids are completely precipitated, collect the solids by suction filtration, and wash them thoroughly with water. After drying, 11.54 g of white benzyl ursolic acid crude product was obtained (crude yield 96.4%). 1 HNMR (CDCl 3 , 300MHz): δ0.67, 0.81, 0.92, 0.97, 1.01, 1.10(each, 3H, s), 0.88(3H, d, J=6.4Hz), 2.30(1H, d, J=11.3Hz, H- 18), 3.24 (1H, dd, J=4.6, 10.8Hz, H-3α), 5.09...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap