Pharmaceutical compositions and methods
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, heterocyclic compound active ingredients, etc., can solve the problems of resistance, expensive treatment, inaccuracy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
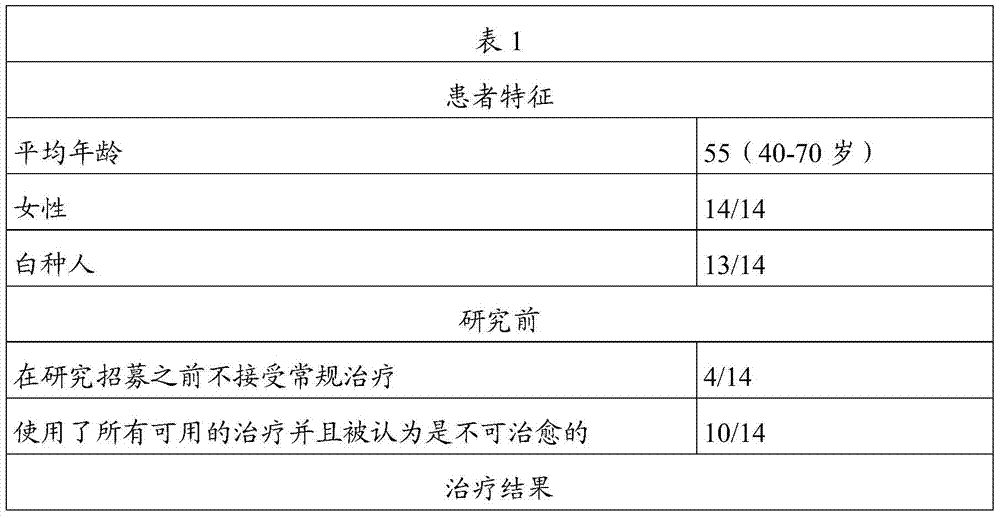
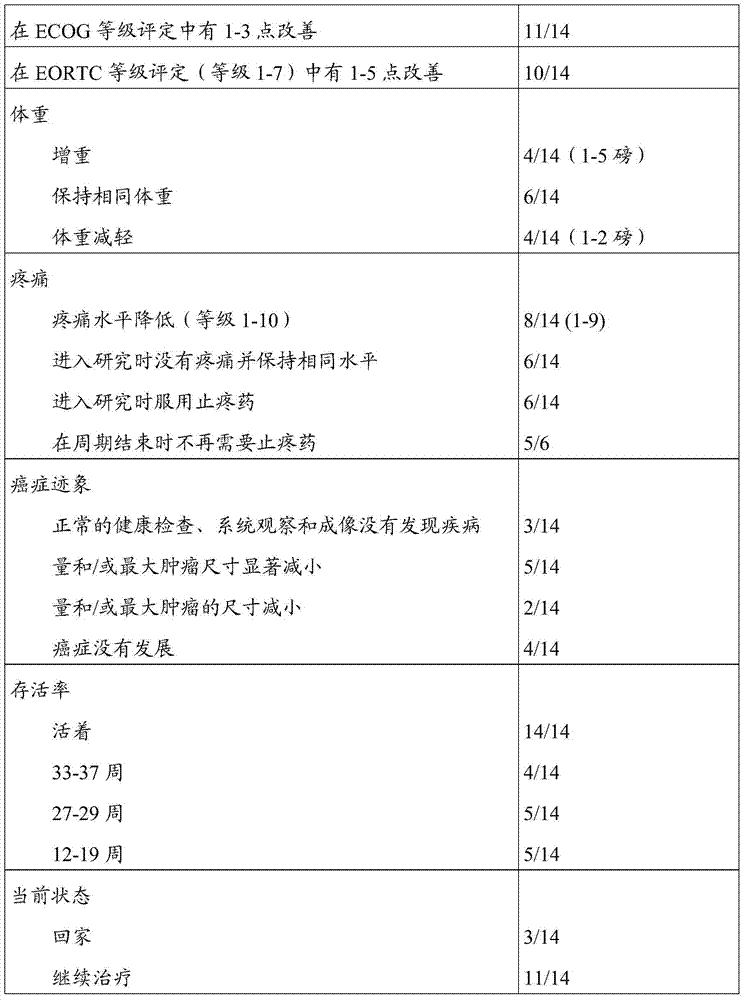
[0059] A clinical study was conducted to evaluate the effectiveness, safety, acceptability and tolerability of combination therapy according to embodiments of the present invention as a therapy for metastatic cancer.
[0060] This combination therapy includes the following:
[0061] (a) Capsules containing melanin (50mcg) and α-methyl-DL-tyrosine (75mg) for oral administration;
[0062] (b) Capsules containing 5,5-diphenylhydantoin (15 mg) and α-methyl-DL-tyrosine (75 mg) for oral administration;
[0063] (c) Capsules containing 5,5-diphenylhydantoin (15 mg) and α-methyl-DL-tyrosine (75 mg) for oral administration;
[0064] (d) Capsules containing rapamycin (0.2 mg) and α-methyl-DL-tyrosine (75 mg) for oral administration;
[0065] (e) a suspension containing rapamycin (0.15mcg), melanotan II (10mcg) and 5,5-diphenylhydantoin (2mg), administered subcutaneously; and
[0066] (f) Suspension containing α-methyl-DL-tyrosine (5 mg) in NaCl bacteriostatic water, administered subc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com