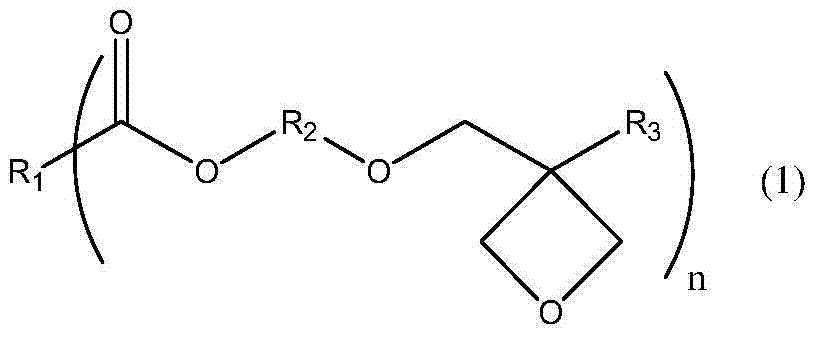
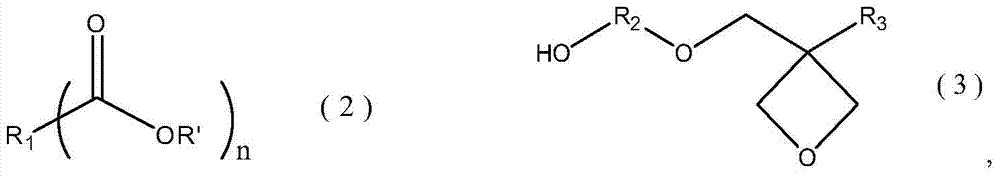
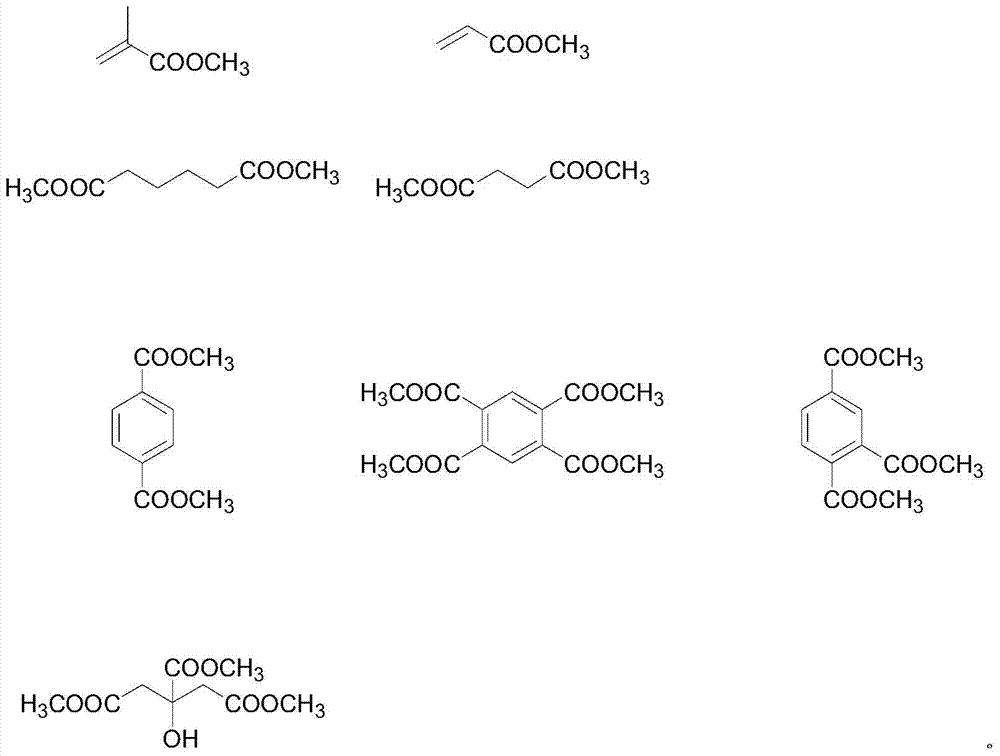
A kind of ester compound containing oxetane group and preparation method thereof
A technology of oxetane group and ester compound, which is applied in the field of oxetane group-containing ester compound and preparation thereof, can solve the problems of reducing production efficiency and the like, and achieves simple preparation, high cationic reactivity, high The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of compound 1
[0034]
[0035]In a glass flask with a volume of 1000 mL having a stirring device, a thermometer, a 60 cm packed tower and a rectifying head, add 160 g (1.0 mol) of raw material 1 and 500 g (5.0 mol) of methyl methacrylate, keep reflux at 110 ° C, and adjust The reflux ratio was 3:1. After the moisture in the system dropped below 500ppm, 6.6g of tetraisopropyl titanate catalyst (accounting for 1% of the total feeding amount) was added, and the reflux was continued for 3 hours. After the reaction, cool down to 70°C, add 25g of water, keep warm at 70°C for half an hour, destroy the catalyst, filter, and concentrate the filtrate to obtain 216g colorless liquid (isolation yield of raw material 1 benchmark: 95%)
[0036] The structure of the product compound 1 was obtained by mass spectrometry and 1 H-NMR confirmed.
[0037] MS(m / e): 229(M+1)
[0038] 1 H-NMR (CDCl 3 , δ(ppm)): 0.96(3H), 1.25(2H), 1.93(3H), 3.65(2H), 4.32(2H), 4.65(4H), 5.58...
Embodiment 2
[0040] Synthesis of compound 2
[0041]
[0042] In a glass flask with a volume of 1000 mL having a stirring device, a thermometer, a 60 cm packed tower and a rectifying head, add 204 g (1.0 mol) of raw material 2, and refer to Example 1 for the remaining operations. After concentration, 261 g of a colorless transparent liquid was obtained (isolation yield based on raw material 2: 96%).
[0043] The structure of the product was confirmed by the following physical property values.
[0044] MS(m / e): 273(M+1)
[0045] 1 H-NMR (CDCl 3 , δ(ppm)): 0.96(3H), 1.25(2H), 1.93(3H), 3.29(2H), 3.54(4H), 3.65(2H), 4.32(2H), 4.65(4H), 5.58(2H ), 6.15(2H).
Embodiment 3
[0047] Synthesis of compound 3
[0048]
[0049] In a glass flask with a volume of 1000 mL having a stirring device, a thermometer, a 60 cm packed tower and a rectifying head, add 248 g (1.0 mol) of raw material 3, and refer to Example 1 for the remaining operations. Concentration gave 294 g of a colorless transparent liquid (isolation yield based on raw material 3: 93%).
[0050] The structure of the product was confirmed by the following physical property values.
[0051] MS(m / e): 317(M+1)
[0052] 1 H-NMR (CDCl 3 , δ(ppm)): 0.96(3H), 1.25(2H), 1.93(3H), 3.29(2H), 3.54(8H), 3.65(2H), 4.32(2H), 4.65(4H), 5.58(2H ), 6.15(2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com