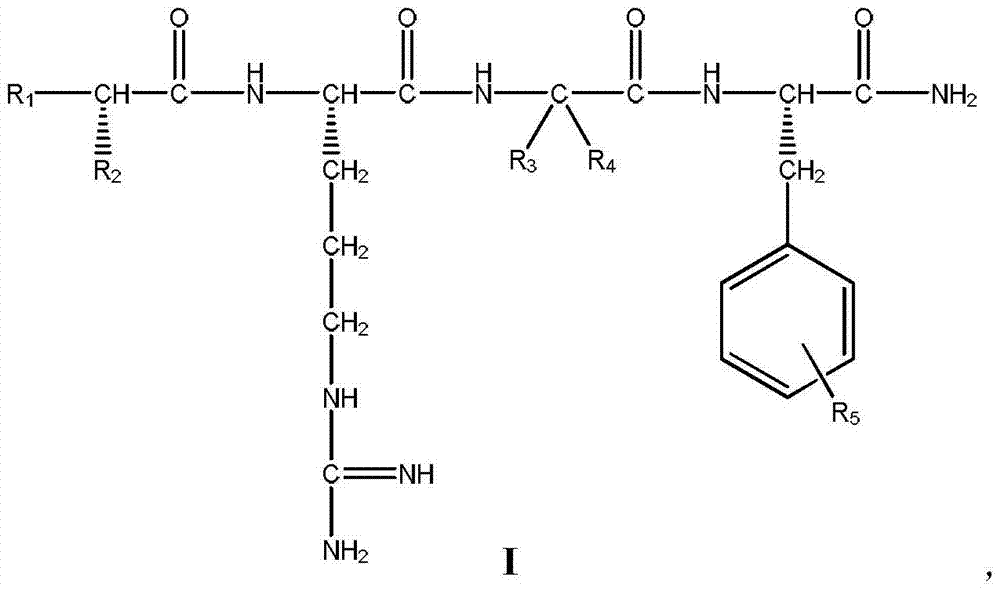
Insect pharyngeal voxin antagonist and use thereof
A pharyngeal-promoting side voxel and antagonist technology, applied in the field of pesticides, can solve the problems of affecting the environment, pesticide residues, etc., and achieve the effects of regulating absorption and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 compound I1 (L-alanyl-L-arginyl-glycyl-L-phenylalaninamide)
[0034]
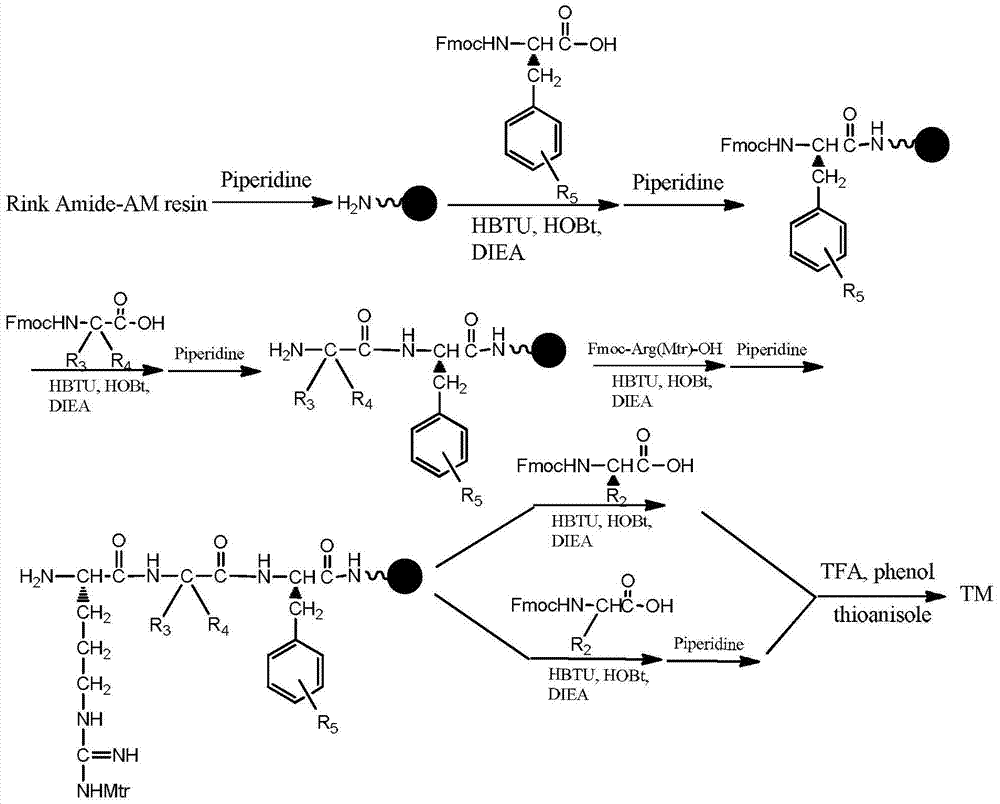
[0035] (1) Resin activation: Weigh 200mg Rink Amide-AM resin, wash 4 times with dichloromethane, add 15ml dichloromethane to swell and activate for 3 hours, wash 4 times with N,N-dimethylformamide, add 20% piperidine Cut with N,N-dimethylformamide solution for 20min, wash 4 times with 5ml N,N-dimethylformamide, wash 4 times with 5ml dichloromethane, and detect with Kaiser's reagent.
[0036](2) Phenylalanine: N,N-dimethylformamide washed 3 times, added 116mg phenylalanine protected by fluorene methoxycarbonyl, 114mg O-benzotriazole-tetramethylureahexa Fluorophosphate (HBTU), 41mg 1-hydroxybenzotriazole (HOBt), 105ul N,N-diisopropylethylamine (DIEA), dissolved in 15ml N,N-dimethylformamide, stirred at room temperature for 2h, 5ml Wash 4 times with N,N-dimethylformamide, add 20% piperidine in N,N-dimethylformamide, cut for 20min, wash 4 times with 5ml N,N-dimethylfor...
Embodiment 2
[0043] The preparation of embodiment 2 compound I5 (L-alanyl-L-arginyl-2,2-difluoroglycyl-L-phenylalaninamide)
[0044]
[0045] (1) Resin activation: Weigh 200mg Rink Amide-AM resin, wash 4 times with dichloromethane, add 15ml dichloromethane to swell and activate for 3h, wash 4 times with N,N-dimethylformamide, add 20% piperidine The N,N-dimethylformamide solution was cut for 20min, washed 4 times with 5ml N,N-dimethylformamide, washed 4 times with 5ml dichloromethane, and detected by Kaiser's reagent.
[0046] (2) Phenylalanine: N,N-dimethylformamide washed 3 times, added 116mg phenylalanine protected by fluorene methoxycarbonyl, 114mg O-benzotriazole-tetramethylureahexa Fluorophosphate (HBTU), 41mg 1-hydroxybenzotriazole (HOBt), 105ul N,N-diisopropylethylamine (DIEA), dissolved in 15ml N,N-dimethylformamide, stirred at room temperature for 2h , washed 4 times with 5ml N,N-dimethylformamide, added 20% piperidine in N,N-dimethylformamide solution for cutting for 20min, w...
Embodiment 3
[0053] The preparation of embodiment 3 compound I9 (L-alanyl-L-arginyl-glycyl-L-4-fluorophenylalaninamide)
[0054]
[0055] (1) Resin activation: Weigh 200mg Rink Amide-AM resin, wash 4 times with dichloromethane, add 15ml dichloromethane to swell and activate for 3h, wash 4 times with N,N-dimethylformamide, add 20% piperidine The N,N-dimethylformamide solution was cut for 20min, washed 4 times with 5ml N,N-dimethylformamide, washed 4 times with 5ml dichloromethane, and detected by Kaiser's reagent.
[0056] (2) Fluorophenylalanine: N,N-dimethylformamide washed 3 times, added 121mg Fmoc-Phe(4-F)-OH, 114mg HBTU, 41mg HOBt, 105ul DIEA, dissolved in 15ml N ,N-dimethylformamide, stirred at room temperature for 2h, washed 4 times with 5ml N,N-dimethylformamide, added 20% piperidine in N,N-dimethylformamide solution for cutting for 20min, 5ml N,N - Wash 4 times with dimethylformamide, 4 times with 5 ml of dichloromethane, and detect with Kaiser's reagent.
[0057] (3) Glycine:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com