A kind of preparation method of alcalcidol intermediate
A saponification and compound technology, applied in the field of preparation of alcalcidol intermediate 1A, can solve the problems of low utilization rate of raw materials, affect product quality, increase the dosage of other materials and reagents, etc., and achieve easy industrial scale-up and improved raw material utilization. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
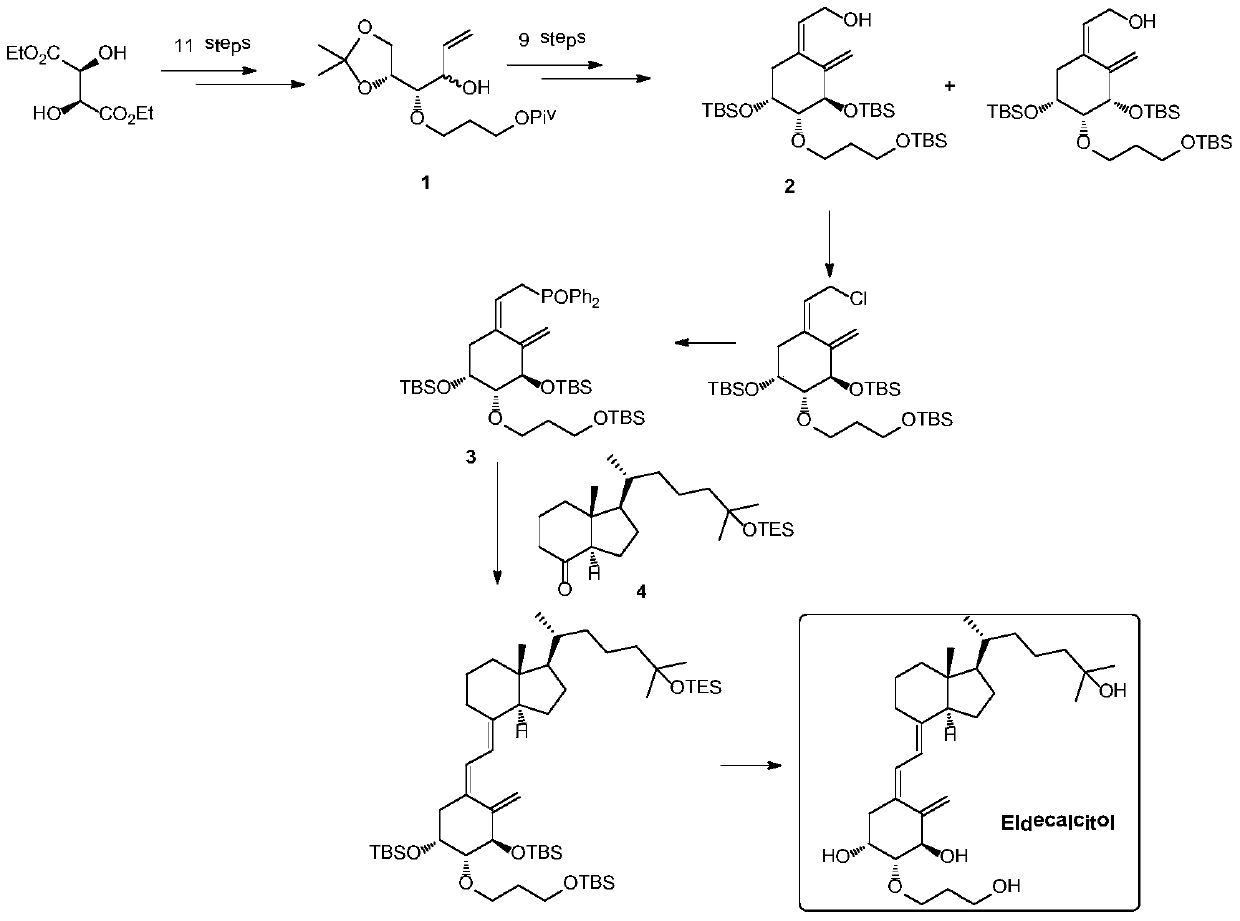
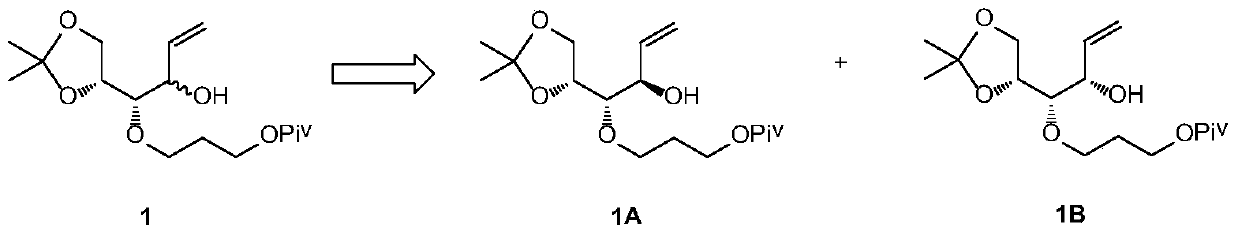
[0035] Example 1 Preparation of Compounds 1A and 7 Using Compound 1 as a Raw Material
[0036]
[0037]Dissolve compound 1 (33g, 100mmol) and ethyl acrylate (20g, 200mmol) in 500mL of n-hexane, add Novozyme lipase 4353g at room temperature, heat to 70°C, keep the temperature and react overnight, sample and filter for HPLC detection, 1B Less than 3%, the reaction is complete. The solid was removed by filtration, the filter cake was washed with 200 mL of n-hexane, and the filtrate was collected and concentrated to obtain 35 g of crude product. The crude product was subjected to silica gel column chromatography to obtain 17g of compound 1A with a yield of 52% and a purity of 97%, and compound 716g with a yield of 48% and a purity of 95%.
[0038] Compound 1A: 1H NMR (400MHz, CDCl3) δ = 5.93 (ddd, 1H, J = 17.4, 10.7, 6.2Hz), 5.34 (dt, 1H, J = 17.4, 1.5Hz), 5.23 (dt, 1H, J = 10.2, 1.5Hz), 4.29–4.09(m, 4H), 4.00(dd, 1H, J=8.4, 6.3Hz), 3.79–3.65(m, 3H), 3.30(dd, 2H, J=6.0, 5.0Hz...
Embodiment 2
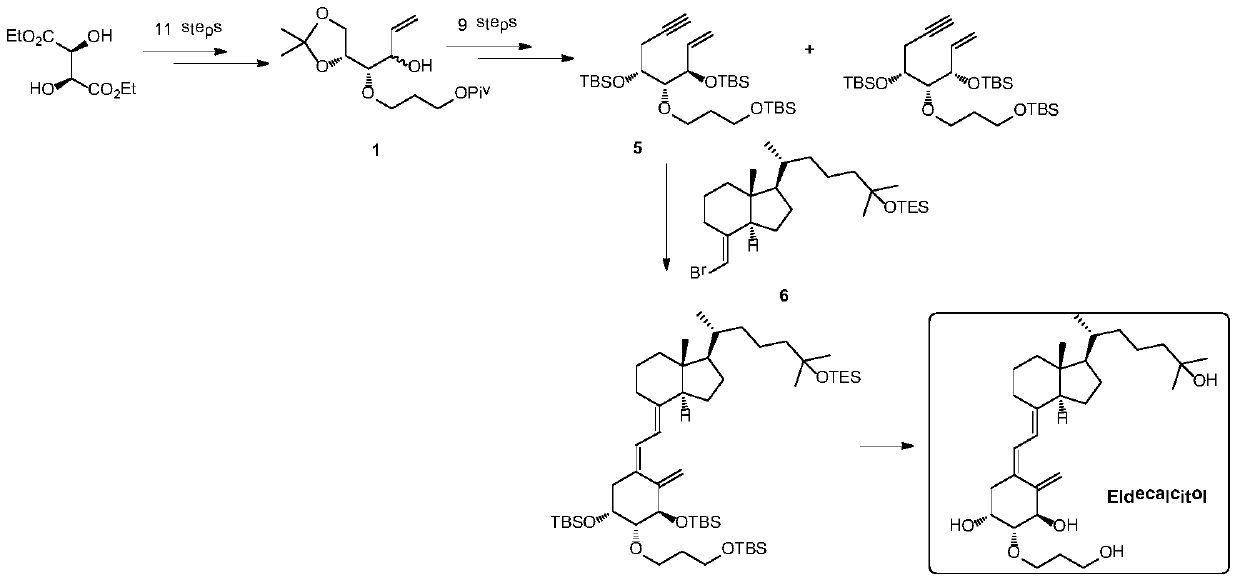
[0042] The preparation of embodiment 2 compound 1B
[0043]
[0044] Compound 7 (15g, 40.3mmol) was dissolved in 100mL tetrahydrofuran and 100mL methanol, and potassium carbonate (11.1g, 80.6mmol) was added after cooling down to zero. After the addition, the temperature was naturally raised to room temperature for 3 hours. React until the spots of raw materials detected by TLC disappear, and the reaction is complete. The reaction was quenched by adding 300 mL of water. Extracted three times with 100 mL of ethyl acetate, combined the organic phases, washed with 200 mL of saturated brine, dried with 200 g of anhydrous Na2SO4, and concentrated to obtain 14 g of crude product. The crude product was chromatographed on a silica gel column to obtain 12.5 g of compound 1B with a yield of 93.9% and a purity of 95%.
[0045] 1B: 1H NMR (400MHz, CDCl3) δ = 5.89 (ddd, 1H, J = 17.4, 10.7, 6.2Hz), 5.35 (dt, 1H, J = 17.4, 1.5Hz), 5.24 (dt, 1H, J = 10.2 ,1.5Hz),4.29–4.09(m,4H),4.01(dd,1...
Embodiment 3
[0047] The preparation of embodiment 3 compound 8
[0048]
[0049] Compound 1B (10g, 30.4mmol) was dissolved in 100mL of dichloromethane, and Dess Martin oxidant (25.6g, 60.6mmol) was added in batches at zero temperature, and stirred for 3 hours after naturally rising to room temperature. After reacting until the spot of raw material disappeared as observed by TLC, it was quenched by adding 500 mL of n-hexane, filtered, the filter cake was washed with 500 mL of n-hexane, and concentrated to obtain 11 g of crude product. Silica gel column chromatography yielded 89.5 g of the compound, with a yield of 95% and a purity of 93%.
[0050] 8: 1H NMR (400MHz, CDCl3): δ = 6.8-36.74 (1H, m), 6.43-6.37 (1H, dd, J = 1.5, 17.37Hz), 5.80-5.76 (1H, dd, J = 1.5, 10.5 Hz), 4.22-4.17(2H,m), 4.0-3.9(2H,m), 3.79–3.65(m,3H), 3.29(dd,2H,J=6.0,5.0Hz), 2.89(d,1H, J=6.3Hz), 1.92(quint, 2H, J=6.3Hz), 1.42(s, 3H), 1.36(s, 3H), 1.20(s, 9H);
[0051] HRMS(EI)m / z calcd for C17H30O6(M+)328.2048,found...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com