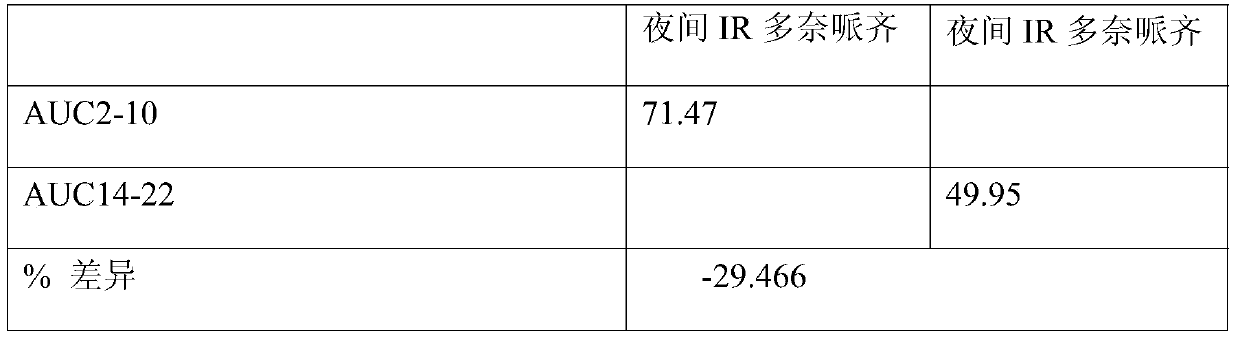
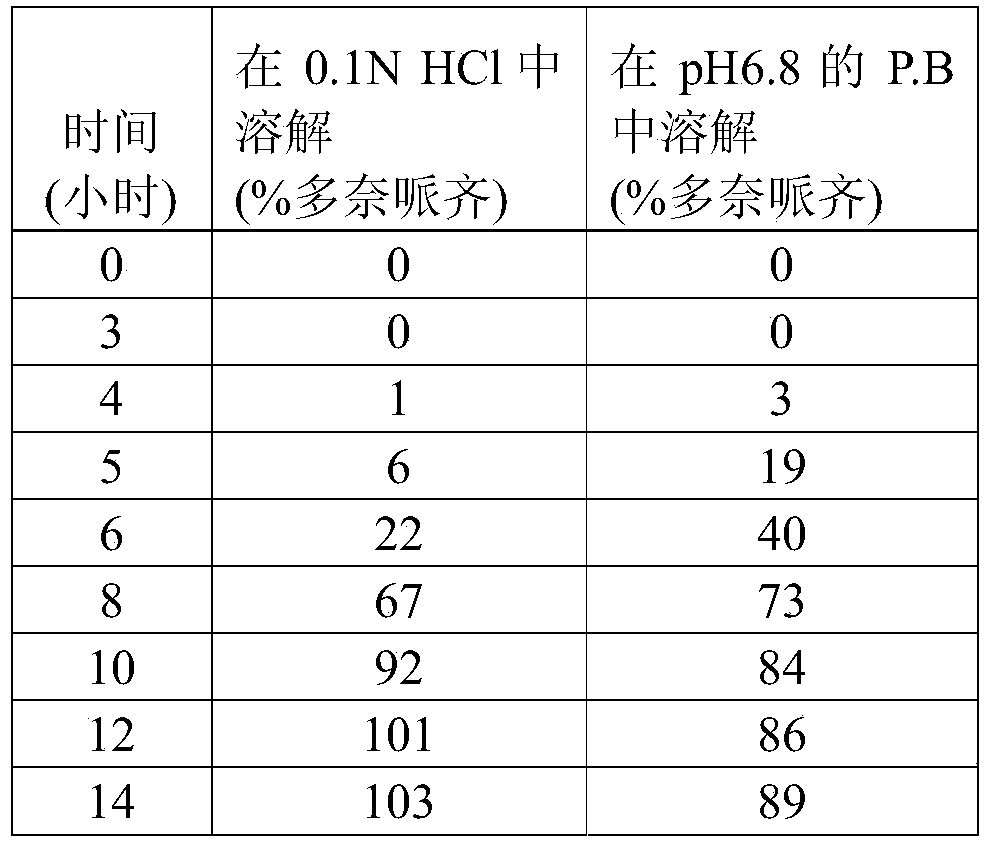
Pharmaceutical compositions of donepezil having specific in vitro dissolution profile or pharmacokinetics parameters
A kind of technology of donepezil and composition, applied in the field of timed release pharmaceutical composition of donepezil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Brief manufacturing process:
[0152] 1 Weigh donepezil HCl, sodium starch glycolate, hydroxypropyl methylcellulose, lactose and hydroxypropyl cellulose, sieving and mixing together.
[0153] 2 Use pure water to granulate the mixture of step 1 with appropriate granulation parameters, and then dry the granules.
[0154] 3 The dried granules are sieved through a suitable sieve.
[0155] The remaining amount of 4-hydroxypropyl methylcellulose, lactose and microcrystalline cellulose is sieved through a suitable sieve and mixed with the granules of step 3.
[0156] 5 Colloidal silicon dioxide and magnesium stearate are sieved through a suitable sieve and mixed with the blend of step 4.
[0157] 6 Compress the lubricating blend of step 5 with a circular punch using appropriate physical parameters.
[0158] 7 Ethyl cellulose, hydroxypropyl methyl cellulose and triethyl citrate were dissolved in a mixture of isopropanol and dichloromethane under continuous stirring.
[0159] 8 Coat the com...
Embodiment 2
[0165] Brief manufacturing process:
[0166] 1 Weigh donepezil HCl, sodium starch glycolate, hydroxypropyl methylcellulose, lactose and hydroxypropyl cellulose, sieving and mixing together.
[0167] 2 Use pure water to granulate the mixture of step 1 with appropriate granulation parameters, and then dry the granules.
[0168] 3 The dried granules are sieved through a suitable sieve.
[0169] The remaining amount of 4-hydroxypropyl methylcellulose, lactose and microcrystalline cellulose is sieved through a suitable sieve and mixed with the granules of step 3.
[0170] 5 Colloidal silicon dioxide and magnesium stearate are sieved through a suitable sieve and mixed with the blend of step 4.
[0171] 6 Compress the lubricating blend of step 5 with a circular punch using appropriate physical parameters.
[0172] 7 Ethyl cellulose, hydroxypropyl methyl cellulose and triethyl citrate were dissolved in a mixture of isopropanol and dichloromethane under continuous stirring.
[0173] 8 Coat the com...
Embodiment 3
[0176] Brief manufacturing process:
[0177] 1 Hardened sugar balls
[0178] 1.1 The sugar balls are sieved using a vibrating screen.
[0179] 1.2 Triethyl citrate and hydroxypropyl methylcellulose are dissolved in a mixture of isopropanol and dichloromethane under continuous stirring.
[0180] 1.3 The sugar spheres are loaded in the fluidized bed processor and fluidized.
[0181] 1.4 All the solutions of step 1.2 are sprayed on the sugar balls at a suitable temperature.
[0182] 1.5 The hardened sugar balls of step 1.4 are dried in a fluidized bed processor at a suitable temperature.
[0183] 1.6 The dried hardened sugar balls of step 1.5 are passed through a suitable sieve and the fines are discharged.
[0184] 2 Drug loading
[0185] 2.1 Add hydroxypropyl cellulose to pure water to obtain a clear solution.
[0186] 2.2 Add donepezil hydrochloride to the solution in step 2.1 to obtain a clear solution.
[0187] 2.3 Add talc to the solution of step 2.2 under continuous stirring and filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com