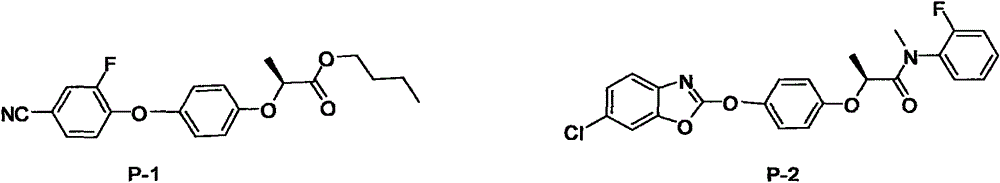
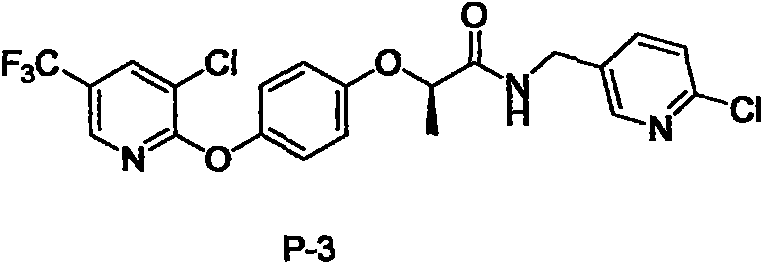
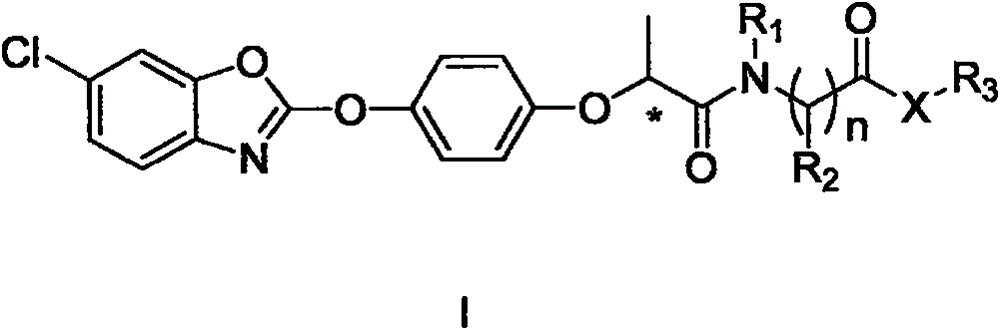
N-substituted alkyl arylxoy phenoxyl propanamide compound with herbicidal activity and preparation and application thereof
An alkylphenoxypropionamide and compound technology, which is applied in the field of weed compounds, can solve the problems of insufficient safety, no description of rice safety, inability to meet agricultural production requirements, etc., and achieves broad herbicidal spectrum and excellent activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 compound 2
[0049]
[0050] (1) Preparation of Intermediate IV
[0051] Add 33.3 grams of intermediate III (prepared according to the method described in patent CN201310606528.9) into a 250 ml four-neck flask, add 150 ml of dichloroethane and 11.8 grams of thionyl chloride. Heated and refluxed for 10 hours. After the reaction is complete, remove the solvent to obtain Intermediate IV, add 150ml of dichloroethane, and cool down to 30°C for use.
[0052] (2) Preparation of Compound 2
[0053] 14 g of ethyl aminoacetate hydrochloride was added to the above-prepared solution, stirred and reacted for 1 hour, 12 g of triethylamine was added dropwise, and a large amount of white solid was precipitated. React for 2 hours. After the reaction was completed, 100 ml of water was added and stirred for 1 hour, then suction filtered and dried to obtain 38 g of the product. Content 96%. Yield 91%.
Embodiment 2
[0054] The preparation of embodiment 2 compound 3
[0055]
[0056] (1) Preparation of N-methylglycyl amino acid methyl ester
[0057] 12.6 grams of glycine methyl ester hydrochloride was added to a 250ml four-necked flask, and 150ml of ethanol and 11 grams of sodium carbonate were added. The temperature was raised to reflux, and 9.5 g of methyl bromide was added dropwise under the reflux state. After the dropwise addition was completed, the reaction was carried out under reflux for 4 hours. After the reaction is complete, cool down to room temperature, filter out the solid with suction, and remove ethanol to obtain N-methylglycinate methyl ester.
[0058] (2) Preparation of compound 3
[0059] The obtained N-methylglycyl amino acid methyl ester was added to the acid chloride solution (prepared by the above method), stirred and reacted at room temperature for 1 hour, 12 g of triethylamine was added dropwise, and a large amount of white solid was precipitated. React for 2...
Embodiment 3
[0060] The preparation of embodiment 3 compound 5
[0061]
[0062] (1) Preparation of Intermediate A
[0063] 11.6 grams of propionaldehyde and 14 grams of hydroxylamine hydrochloride were added to a 250 ml four-necked reaction flask, 100 ml of ethanol and 8 grams of sodium hydroxide were added, and the temperature was raised to reflux for 4 hours to complete the reaction. Ethanol was removed to obtain 13 grams of propionaldehyde oxime.
[0064] Add 11 grams of propionaldoxime into a 250 ml four-neck flask, add 100 ml of dichloroethane, and dropwise add 30 grams of 2-bromoacetyl bromide. After the dropwise addition, continue to add 15 grams of triethylamine dropwise. After the dropwise addition, the stirring reaction was continued for 4 hours. After the reaction was completed, 25.6 g of Intermediate A-1 was obtained, with a content of 97%.
[0065] Add 14.7 grams of phthalimide, 4 grams of sodium hydroxide, and 100 ml of N,N-dimethylformamide into a 250 ml four-neck rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com