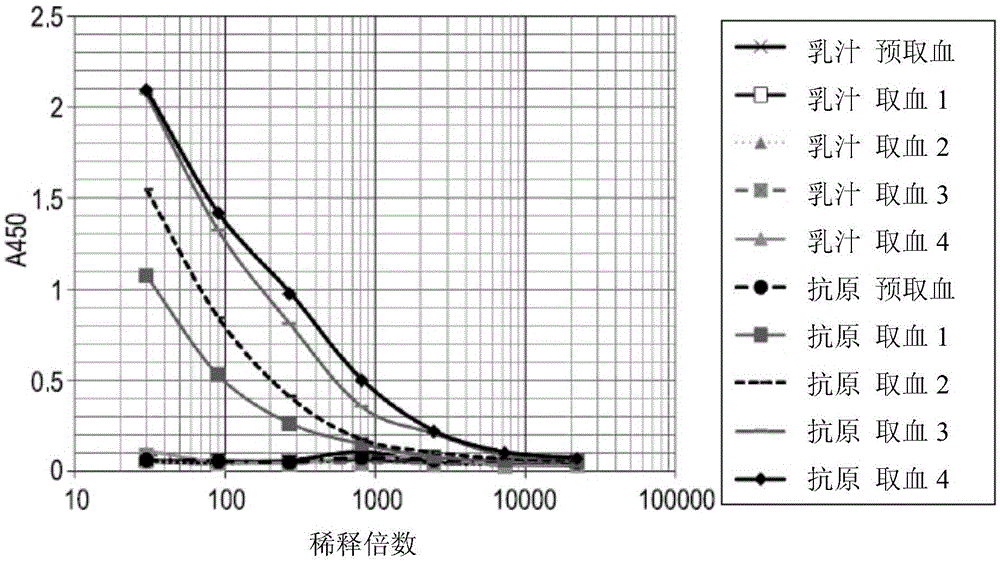
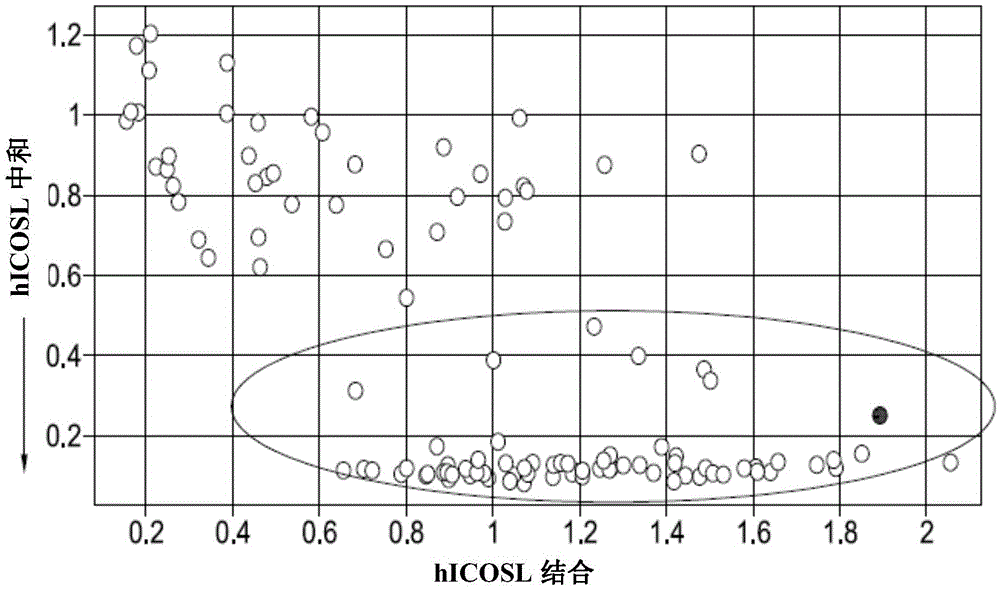
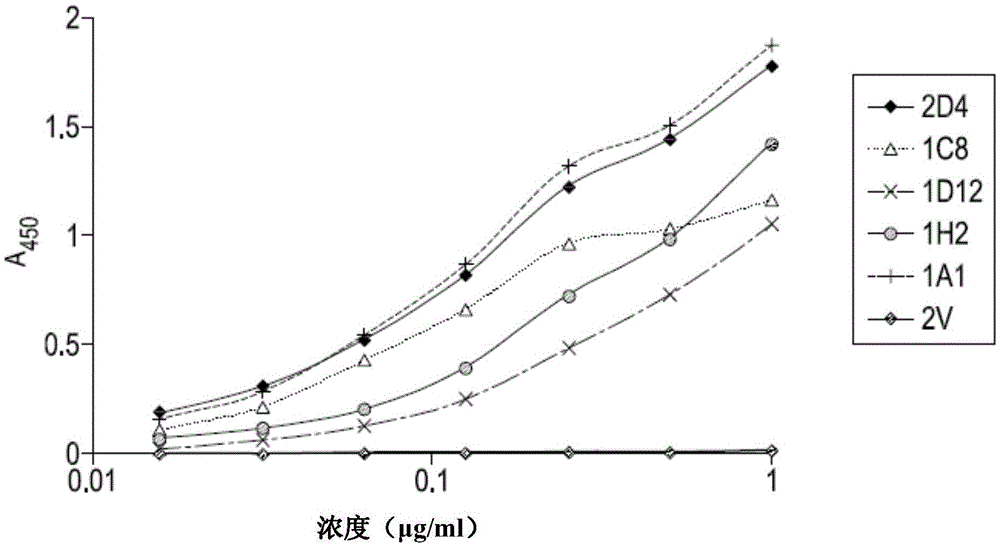
Isolation of therapeutic target specific VNAR domains to ICOSL
A specific, targeted analyte technology, applied in the field of neoantigen receptor domains, which can solve the problems of diversity limitation and achieve high affinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0072] In one embodiment of the present invention, an antigen-specific antigen-binding molecule is provided, which contains the amino acid sequence shown in formula (I)
[0073] A-X-B-Y-C(I)
[0074] in,
[0075] A- is SEQ ID NO: 1 or SEQ ID NO: 4
[0076] X is a CDR1 region of 5, 6 or 7 amino acid residues
[0077] B - for SEQ ID NO: 2 or SEQ ID NO: 5
[0078] Y is a CDR3 region of 8, 9, 10, 11, 12, 13, 14, 15, 16 or 17 amino acid residues
[0079] C- is SEQ ID NO: 3 or SEQ ID NO: 6
[0080] or sequences at least 50% homologous to them,
[0081] Among them, SEQ ID NO: 1 is TRVDQTPRTATKETGESLTINCVLTDT, TRVDQTPRTATKETGESLTINCVVTGA
[0082] SEQ ID NO: 2 is TSWFRKNPGTTDWERMSIGGRYVESVNKGAKSFSLRIKDLTVADSATYYCKA or TSWFRKNPGTTDWERMSIGGRYVESVNKGAKSFSLRIKDLTVADSATYICRA
[0083] SEQ ID NO: 3 is DGAGTVLTVN
[0084] SEQ ID NO: 4 is ASVNQTPRTATKETGESLTINCVLTDT
[0085] SEQ ID NO: 5 is TYWYRKNPGSSNQERISISGRYVESVNKRTMSFSLRIKDLTVADSATYYCKA or TYWYRKNPGSSNQERISISGRYVESVNKRTMSFSLRIKDL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com