Methods and compositions comprising a combination of a vegf antagonist and an anti-ctla-4 antibody
A-CTLA-4, antagonist technology, applied in chemical instruments and methods, antibody mimics/scaffolds, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1. Antitumor Effect of Combination of Anti-CTLA-4 Antibody and VEGF Antagonist in Mouse Early Treatment Tumor Model
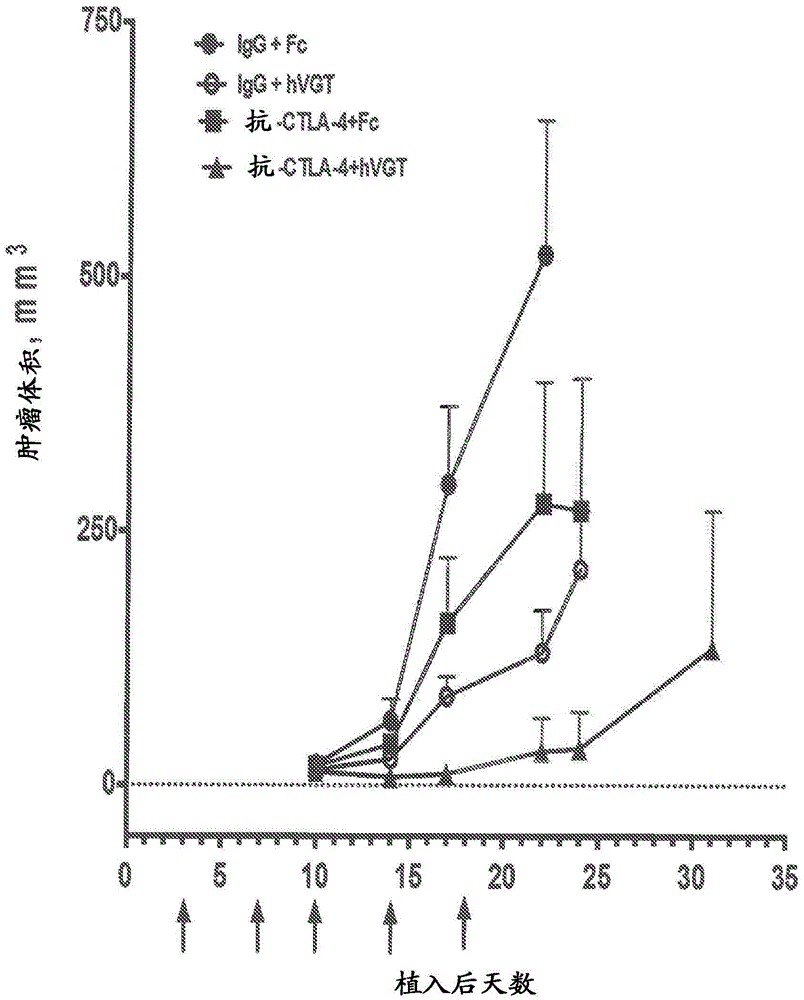
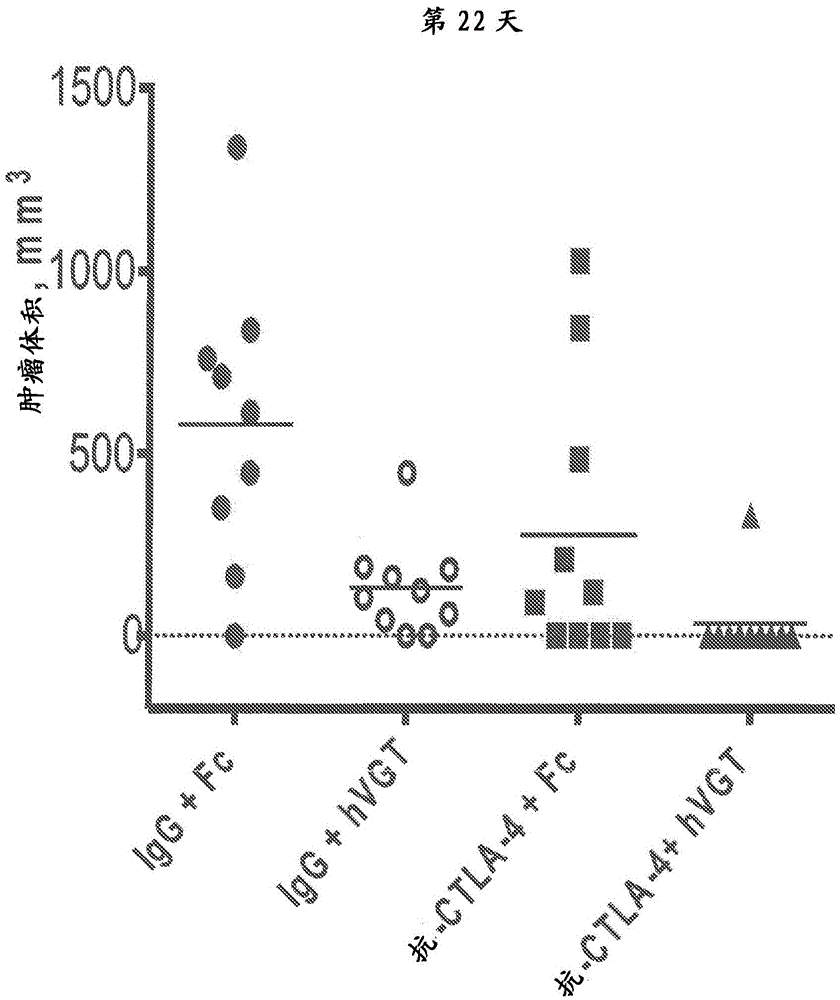
[0088] An early treatment tumor model was developed to test the efficacy of the combination of anti-CTLA-4 antibody and VEGF antagonist. In this model, the combination therapy was administered shortly after tumor implantation. The anti-CTLA-4 antibody used in this experiment was the anti-mouse CTLA-4 IgG2b clone "9D9" (BioX Cell, West Lebanon, NH, catalog number BE0164). The VEGF antagonist used in this experiment was aflibercept (a VEGF receptor-based chimeric molecule, also known as "VEGF-Trap" or "VEGFR1R2-FcΔC1(a)", a full description of which is provided elsewhere herein. describe).
[0089] For this experimental model, 1.0x10 6 Colon-26 tumor cells were implanted into mice. Beginning on day three and prior to the establishment of measurable tumors, treat mice with either monotherapy or combination therapy or a control combination, as li...
Embodiment 2
[0098] Example 2. Tumor Immunization of Animals Previously Treated with a Combination of Anti-CTLA-4 Antibody and VEGF Antagonist in a Mouse Early Treatment Tumor Model
[0099] As a follow-up to Example 1, tumor-free animals were selected from each treatment group (see Tables 1 and 2) and treated with 1.0x10 6 Colon-26 tumor cells were challenged again. Also included in this experiment as a control were naive animals that did not receive tumor challenge or treatment. The individual treatment groups used for this follow-up experiment are summarized in Table 3.
[0100] table 3
[0101]
[0102] At day 60 after the initial tumor cell implantation, animals from each treatment group were implanted with 1.0x10 6 Colon-26 tumor cells (see Example 1). Tumor volume and survival were assessed on days 14 and 32 after rechallenge of the tumors. Summarize the results in Figure 4A and 4B middle. As shown, anti-CTLA-4-only and VEGFTrap+anti-CTLA-4 animals remained tumor-free th...
Embodiment 3
[0104]Example 3. Anti-Tumor Effect of Combination of Anti-CTLA-4 Antibody and VEGF Antagonist in Mouse Late Treatment Tumor Model
[0105] In Example 1, mice received therapeutic treatment shortly after tumor implantation. In this example, by contrast, treatment is purposefully delayed until the tumor is established. Experiments were performed as follows: On day 0, 1.0x10 6 Colon-26 tumor cells were implanted into mice. On day 11, after the tumor has grown to approximately 60mm 3 , mice with the same mean tumor size were randomly grouped and treated with either monotherapy or combination therapy or a control combination, as used in Example 1 (see Table 1). Each therapy was administered at 5 different time points (ie injections on days 11, 15, 17, 21 and 25) over a two-week period.
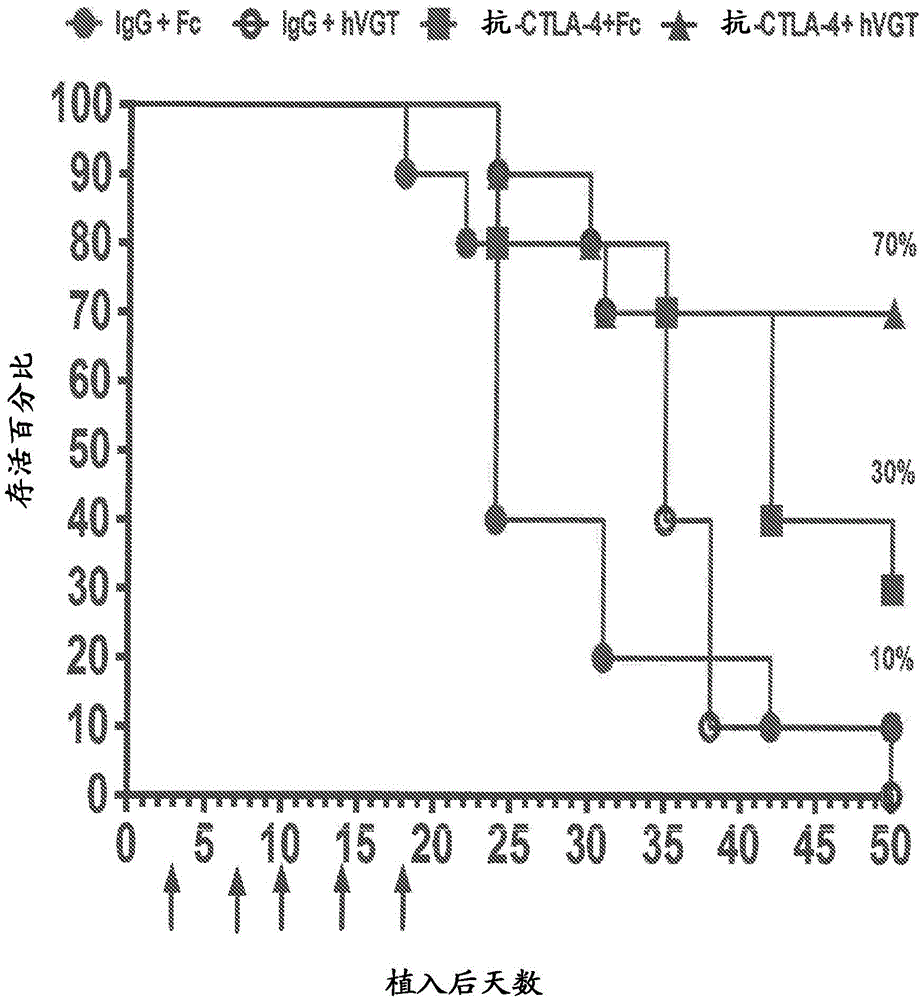
[0106] Animals in each treatment group were evaluated based on tumor incidence, tumor volume, median survival time, and number of tumor-free animals up to day 70. Summarize the extent of tumor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap