Method and composition for enhancing anti-angiogenic therapy
A technology of angiogenesis inhibition and composition, which is applied in the direction of drug combination, antineoplastic drugs, pharmaceutical formulations, etc., and can solve problems such as not showing sufficient efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
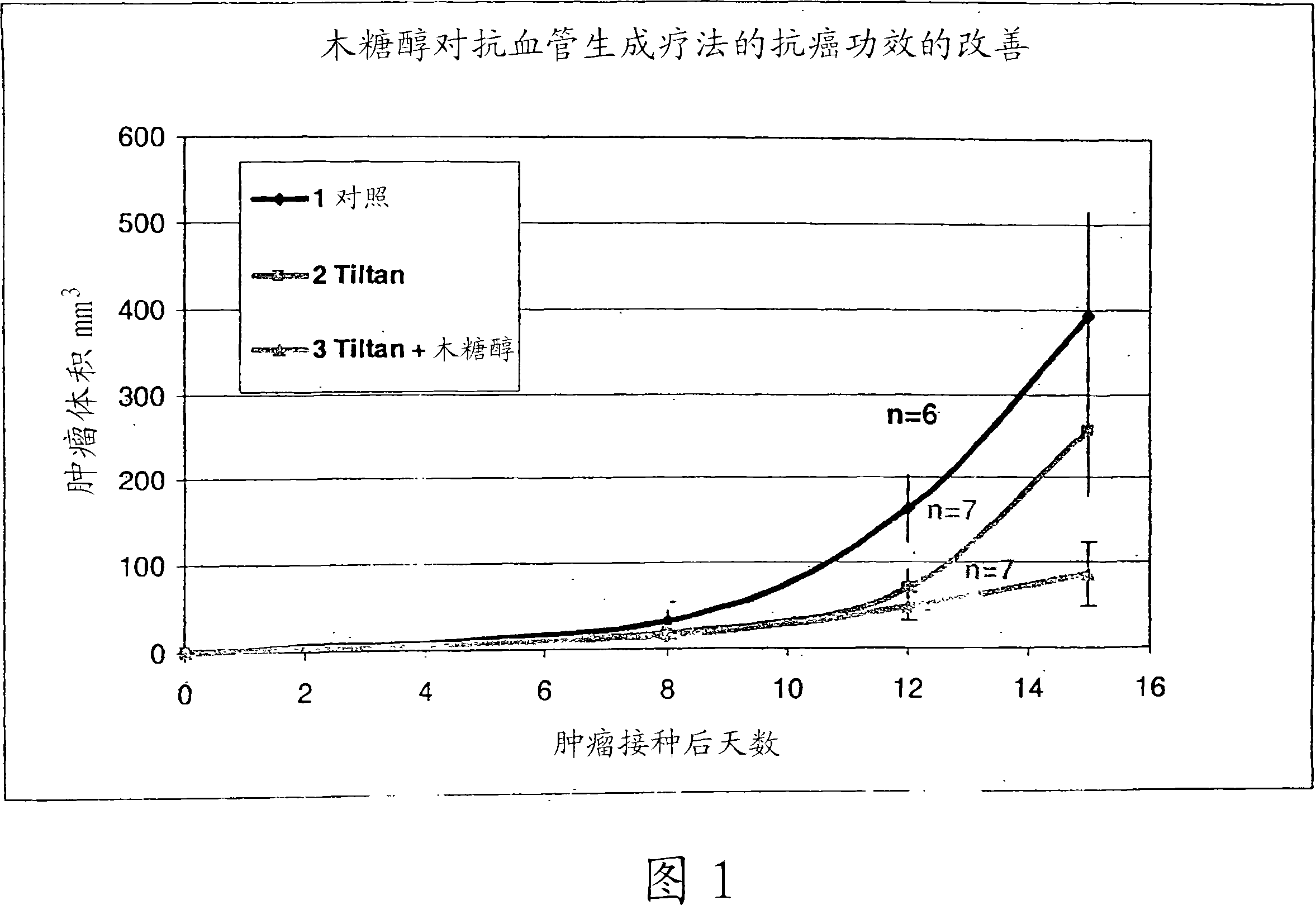
[0087] Cyclophosphamide-resistant breast cancer cells of the EMT-6 / CTX cell line were thawed, grown in tissue culture plates, and injected subcutaneously (s.c.) (10 6 cells / ml) into the rear flank of 27 g CB6F1 male mice.
[0088] The anti-angiogenic therapy (hereby defined as "4x4") consisted of periodic combinations of the drugs detailed in Table 1. The efficacy of 4x4 treatment with xylitol (group 3) or 4x4 treatment without xylitol (group 2) was compared.
[0089] The mice were divided into 3 groups, 7 in each group. Five days after tumor inoculation, the 4x4 group and the 4x4+xylitol group received intraperitoneal injections of corresponding doses (as per the mg / Kg doses shown in Table 1 for each group). The agent containing menadione, cyclophosphamide and diclofenac (= complete combination) is administered twice a week (Sunday and Wednesday) within 4 weeks after inoculation, and during the same period, during the remaining 4 days of the week (Except Saturday) A dose c...
Embodiment 2
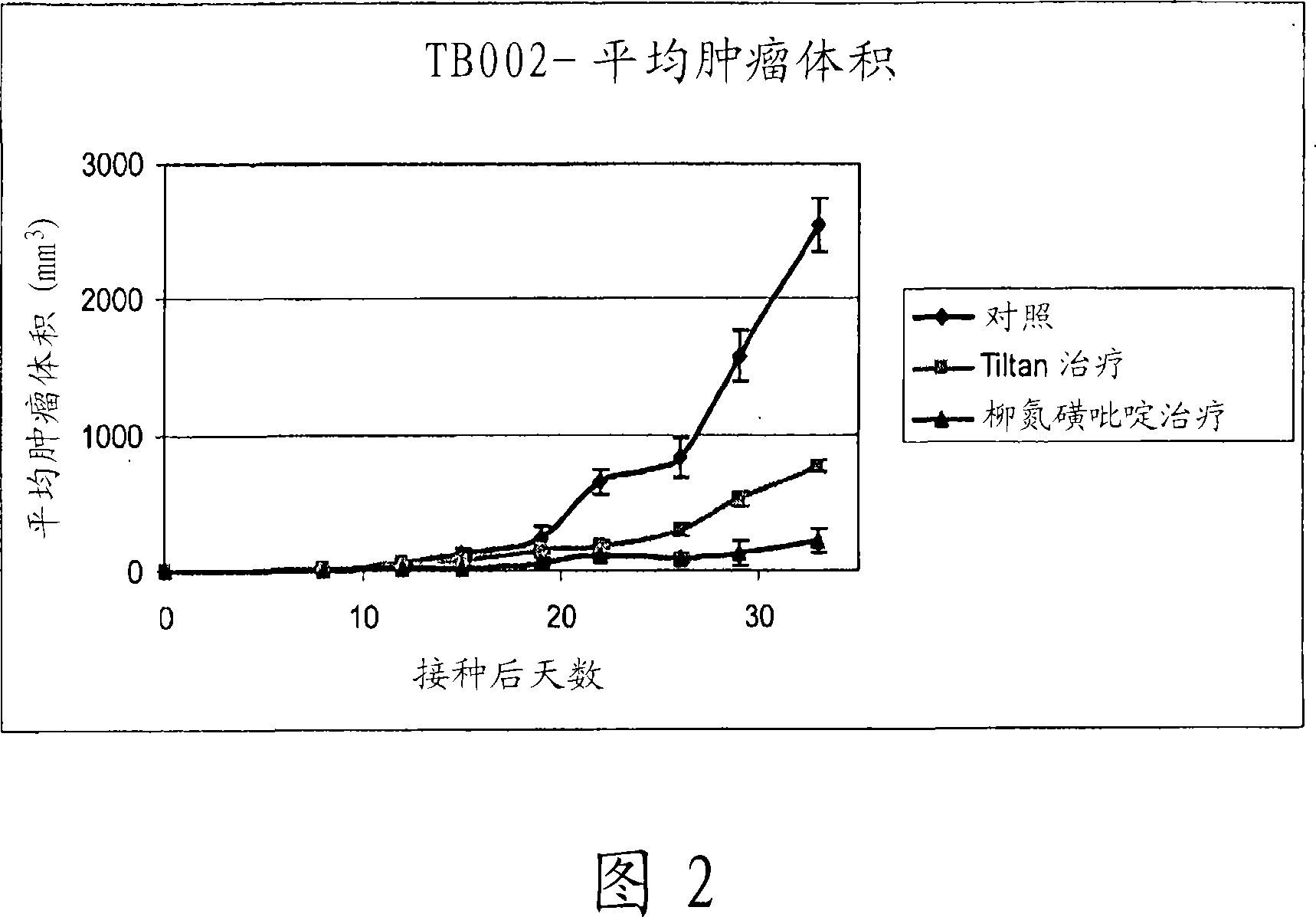
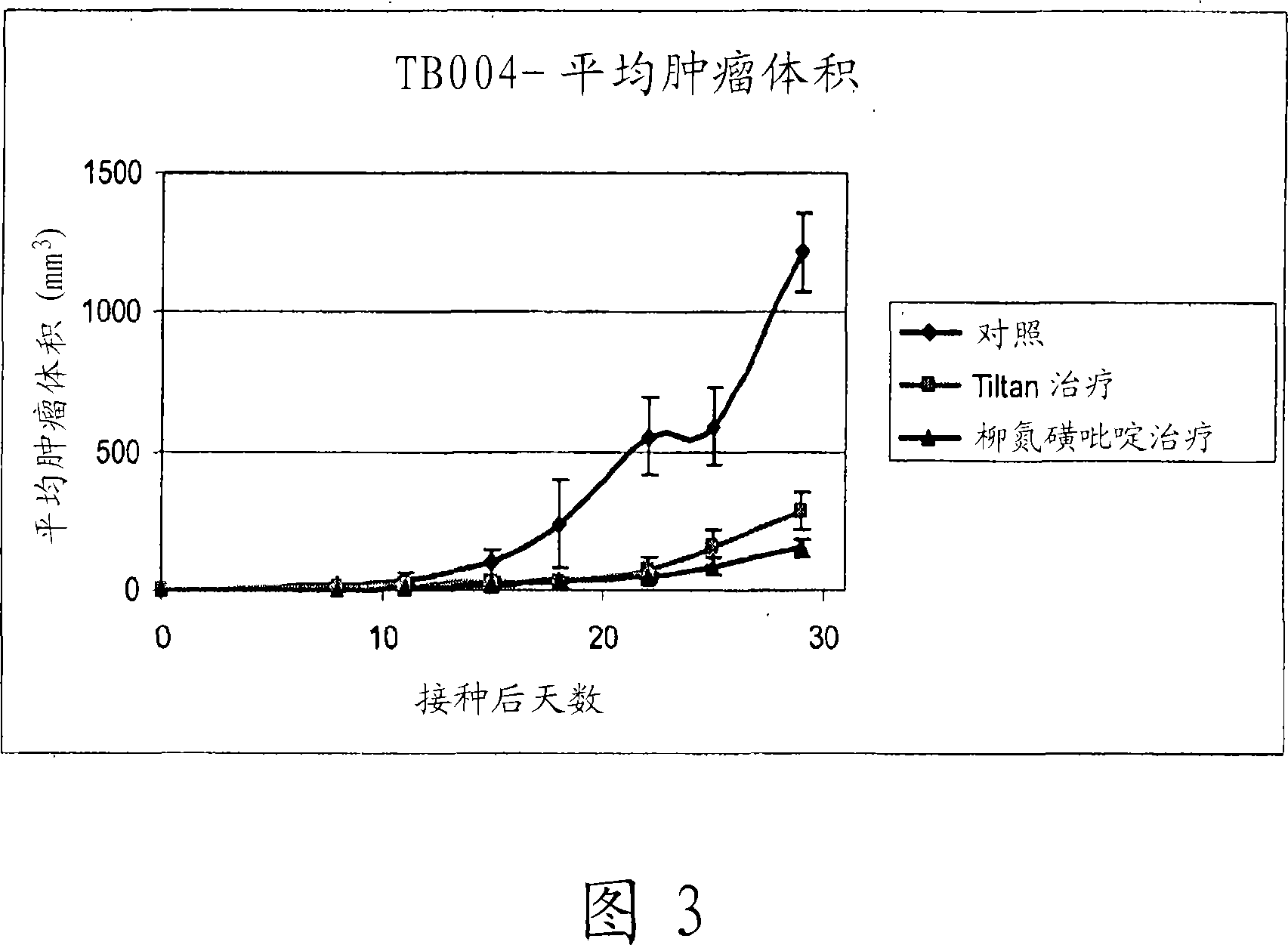
[0094] Experiments Demonstrating the Efficacy of Adding Sulfasalazine to Tiltan Formulations
[0095] The term "Tiltan formulation" or "Tiltan" as used here and throughout is the treatment regimen described in Table 1 Group 3 ("4x4"+xylitol; complete combination+xylitol).
[0096] Experiments discussed below show that the addition of sulfasalazine to Tiltan formulations results in enhanced tumor suppression in a murine in vivo model.
[0097] program
[0098] In the following experiments, different drug combinations for inhibiting tumor growth in mice were tested in vivo. The drug combination was compared with the control group (receiving the vehicle containing only the inactive ingredients) and the Tiltan group (receiving the Tiltan drug combination).
[0099] Inoculation: subcutaneously inject 3.5 × 10 5 mouse breast cancer cells (EMT 6 / CTX). Mice were then labeled and grouped.
[0100] Tumor measurements: Tumor dimensions were measured and graphed twice a week. The ...
Embodiment 3
[0120] Case study: Ovarian cancer patient with lung and liver metastases.
[0121] The patient was a 60-year-old ovarian cancer patient with lung and liver metastases.
[0122] Time 0: Ovary with metastatic adenocarcinoma (Stage IV); TAH+BSO
[0123] Time 0+5 months: adjuvant therapy with carboplatin and taxol. Following an increase in CA-125 labeling (see Figure 4), the patient was switched to TiltAn therapy (performance status-0).
[0124] Time 0+30 months: Start TiltAn treatment (50% dose at weeks 1 and 2; 75% dose at weeks 3 and 4; full dose after week 5).
[0125] The 60% aqueous solution of xylitol of 50ml, this aqueous solution contains following reagent:
Cyclophosphamide 400mg
Diclofenac 200mg
Vitamin K 3 140mg
[0126] The 60% aqueous solution of xylitol of 50ml, this aqueous solution contains following reagent:
Vitamin K 3 →140mg
[0127] Results (see Figures 4 and 5 for details):
[0128] Tumor markers: A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com