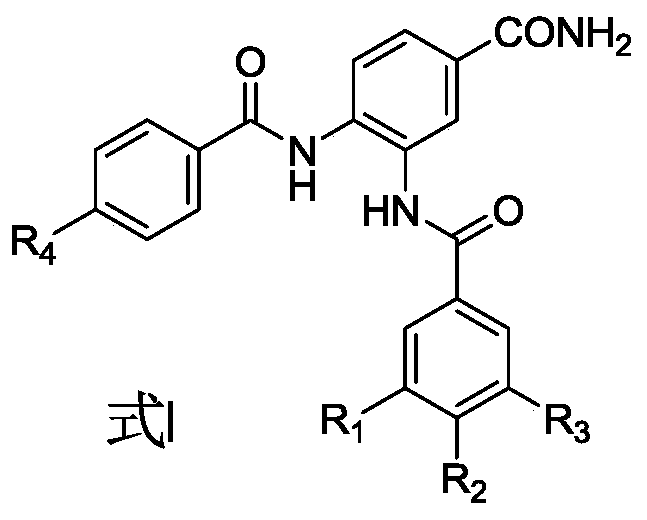
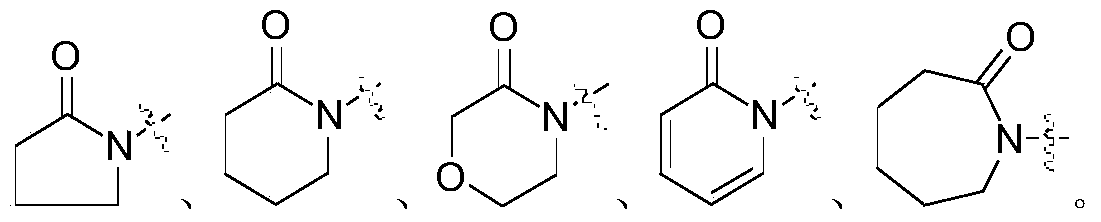
A kind of 3,4-dibenzamidobenzamide derivative and its preparation method and application
A dibenzamide-based benzamide and benzamide-based technology, which is applied in the field of medicinal chemistry and can solve problems such as use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: N-(5-carbamoyl-2-(4-(2-oxopyridin-1(2H)-yl) benzamido) phenyl)-4-methoxybenzamide ( Compound 1) Preparation
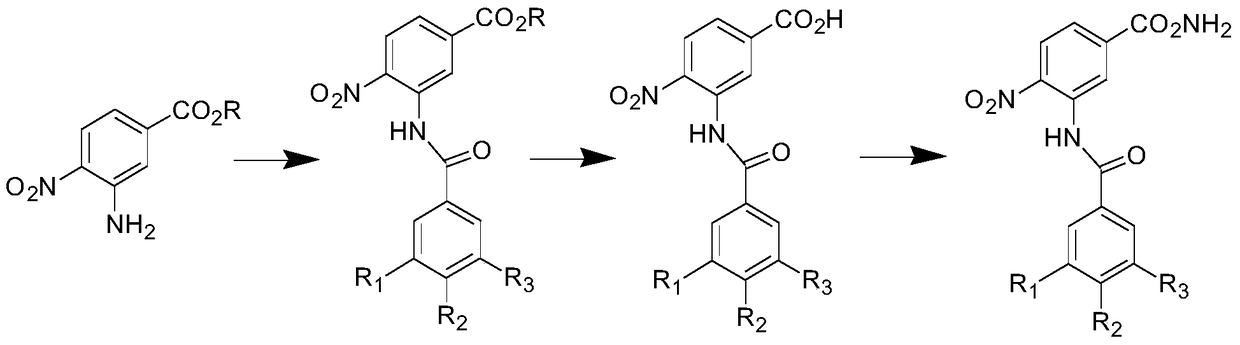
[0075] Preparation of 4-(2-oxopyridin-1(2H)-yl)benzoic acid
[0076]
[0077] Add 400mL of absolute ethanol dried over 3A molecular sieves, 33.0g (0.2mol) of 4-carboxyphenylboronic acid, 8mL of concentrated sulfuric acid, reflux at 105-110°C for 24h, and TLC (CHCl 3 / MeOH=10 / 1) detects that there are very few raw materials, and the ethanol is evaporated under reduced pressure to obtain a white solid, which is added to 500 mL of water, filtered, and saturated with NaHCO 3 The solution was washed with 250 mL, washed with water until neutral, and dried to obtain 33.5 g of 4-ethoxycarbonylphenylboronic acid (yield 86.3%).
[0078] Sequentially add dry CH 2 Cl 2 200mL, 9.5g (0.1mol) of 2-hydroxypyridine, 20.0g (0.1mol) of copper acetate monohydrate, add Et 3 N 100mL, 19.7g (0.1mol) of 4-ethoxycarbonylphenylboronic acid, 20.0g of powdered 4A mol...
Embodiment 2
[0095] Embodiment 2: the preparation of compound 2
[0096] With 3,4-dimethoxybenzoic acid instead of 4-methoxybenzoic acid, it was prepared according to the method of Example 1: N-(5-carbamoyl-2-(4-(2-oxopyridine- 1(2H)-yl)benzamido)phenyl)-3,4-dimethoxybenzamide (compound 2, mp: 278-282°C; ESI-MS: m / z 513 [M+1 ] + ).
Embodiment 3
[0097] Embodiment 3: the preparation of compound 3
[0098] With 3,4,5-trimethoxybenzoic acid instead of 4-methoxybenzoic acid, it was prepared according to the method of Example 1: N-(5-carbamoyl-2-(4-(2-oxopyridine -1(2H)-yl)benzamido)phenyl)-3,4,5-trimethoxybenzamide (compound 3, mp: 265-267°C; ESI-MS: m / z 543 [M +1] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com