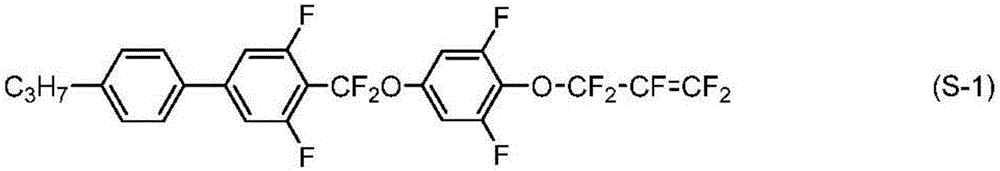
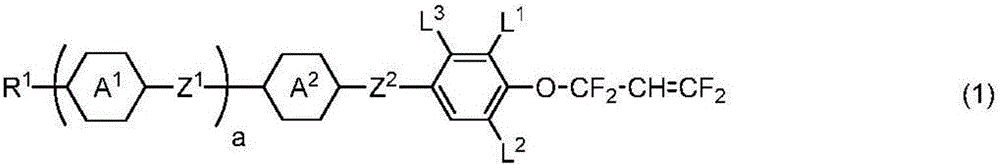
Liquid crystal compound having 1,1,3,3-tetrafluoroallyloxy group, liquid crystal composition, and liquid crystal display element
一种液晶组合物、化合物的技术,应用在有机化学、液晶材料、非线性光学等方向,能够解决相容性不够高、介电常数各向异性大、稳定性不够高等问题,达到响应时间短、介电常数各向异性大、电压保持率大的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0232] Composition (1) is prepared by a method such as dissolving essential components at high temperature. Additives can also be added to this composition according to the use. Examples of additives are optically active compounds, polymerizable compounds, polymerization initiators, antioxidants, ultraviolet absorbers, light stabilizers, heat stabilizers, defoamers, pigments, and the like. Such additives are widely known to those skilled in the art and are described in the literature.
[0233] Composition (1) may also further contain at least one optically active compound. The optically active compound has an effect of preventing reverse twist by inducing a helical structure in liquid crystal molecules to impart a necessary twist angle. Preferable examples of the optically active compound include the following compound (Op-1) to compound (Op-18).
[0234]
[0235] In compound (Op-18), ring F is 1,4-cyclohexylene or 1,4-phenylene, R 21 is an alkyl group having 1 to 10 ca...
Embodiment 1
[0290] [Example 1] 4-((3,5-difluoro-4-((1,1,3,3-tetrafluoroallyl)oxy)phenoxy)difluoromethyl)-3,5 -Synthesis of difluoro-4'-propyl-1,1'-biphenyl (No.1-2-73)
[0291]
[0292] 4-((3,5-difluoro-4'-propyl-[1,1'-biphenyl]-4-yl)difluoromethoxy base)-2,6-difluorophenol (2.00g, 4.69mmol) and potassium carbonate (2.16g, 15.62mmol), and 1,3-dibromo-1,1,3,3-tetrafluoropropane (2.15g , 7.83mmol) was stirred in acetonitrile at 65°C for 3 hours. The reaction liquid was poured into water, and extracted with toluene. The organic layer was washed with water and saturated brine, dried over magnesium sulfate, and the solvent was distilled off with an evaporator. The residue was purified by silica gel chromatography and recrystallization, thereby obtaining compound (No. 1-2-73) (yield 62%).
[0293] 1 H-NMR (CDCl 3 )δ7.50-7.48(m, 2H), 7.30-7.29(m, 2H), 7.22-7.20(m, 2H), 7.00-6.97(m, 2H), 5.00-4.93(m, 1H), 2.66- 2.63(m, 2H), 1.68(sex, J=7.6Hz, 2H), 0.97(t, J=7.3Hz, 3H).
[0294] The phy...
Embodiment 2
[0295] [Example 2] 4-(3,5-difluoro-4-((1,1,3,3-tetrafluoroallyl)oxy)phenyl)-4'-propyl-1,1' -Synthesis of bis(cyclohexane) (No.1-2-40)
[0296]
[0297] Using 2,6-difluoro-4-(4'-propyl-[1,1'-bis(cyclohexane)]-4-yl)phenol synthesized by the method described in JP-A-2007-277127 (3.00 g, 8.92 mmol), the compound (No.1-2-40) was obtained by the same method as in Example 1 (yield 75%).
[0298] 1 H-NMR (CDCl 3 )δ6.83-6.79 (m, 2H), 4.99-4.91 (m, 1H), 2.44-2.38 (m, 1H), 1.91-1.84 (m, 4H), 1.78-1.71 (m, 4H), 1.38- 1.23(m, 5H), 1.16-0.95(m, 8H), 0.89-0.82(m, 5H).
[0299] The physical properties of the compound (No.1-2-40) are as follows. Phase transition temperature: C 46.0N 172I. Upper limit temperature (NI) = 116°C; dielectric constant anisotropy (Δε) = 6.1; optical anisotropy (Δn) = 0.104; viscosity (η) = 33.5mPa·s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| superconducting critical temperature | aaaaa | aaaaa |
| superconducting critical temperature | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com