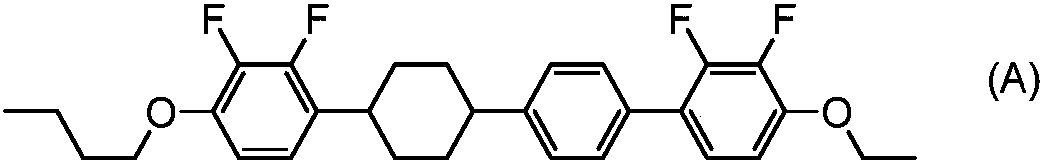
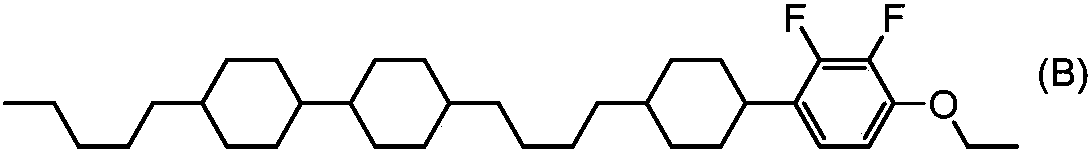
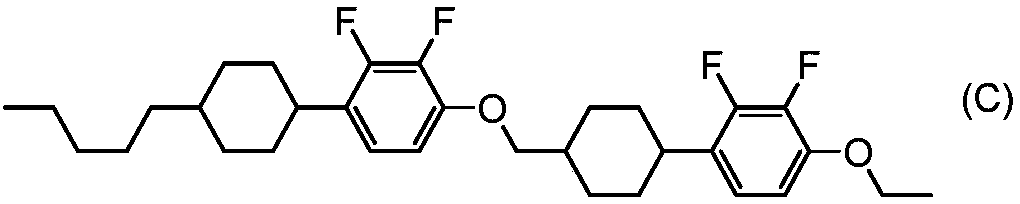
Tetracyclic liquid crystalline compound having two atom-bonded group and 2,3-difluorophenylene, liquid crystal composition and liquid crystal display element
A compound and phenylene technology, applied in the field of liquid crystal display elements, can solve problems such as unclear performance of liquid crystals, and achieve the effects of short response time, wide temperature range, and large contrast ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0238] 1. Examples of compound (1)
[0239] The present invention is illustrated in more detail by way of examples. The Examples are typical examples, and therefore the present invention is not limited by the Examples. Compound (1) was synthesized by the following procedure. The synthesized compounds were identified by nuclear magnetic resonance (Nuclear Magnetic Resonance, NMR) analysis and other methods. The physical properties of the compound or composition, and the characteristics of the device were measured by the following methods.
[0240] NMR analysis: For measurement, DRX-500 manufactured by Bruker BioSpin was used. exist 1 In the measurement of H-NMR, the sample is dissolved in CDCl 3 and other deuterated solvents, and measured at room temperature, 500 MHz, and accumulated times 16 times. Tetramethylsilane was used as an internal standard. exist 19 In the determination of F-NMR, the CFCl 3 It was used as an internal standard, and it carried out by accumulati...
Synthetic example 1
[0274] Synthesis of Compound (No.121)
[0275]
[0276] Step 1:
[0277] The tetrahydrofuran (Tetrahydrofuran, THF) (100mL) solution of the compound (T-2) (15.5g, 27.5mmol) synthesized by a known method was cooled to -60°C, and potassium tert-butoxide (3.08g, 27.5mmol) was added dropwise. ), and stirred for 1 hour. Thereto, a THF (100 mL) solution of compound (T-1) (6.7 g, 25 mmol) synthesized by a known method was added dropwise, and the mixture was returned to room temperature while stirring. The reaction mixture was poured into water, subjected to usual post-treatment, and purified by silica gel chromatography. Solmix A-11 (100 mL), toluene (50 mL) and 6N hydrochloric acid (20 mL) were added to the purified product (8.3 g, 17.6 mmol; 70%), and heated to reflux for 4 days. It purified by column chromatography and recrystallization after usual post-processing, and obtained compound (No. 121) (4.6 g, 9.75 mmol; 55%).
[0278] 1 H-NMR (CDCl 3 ;δppm): 6.84(1H,dt,7.5Hz,2...
Synthetic example 2
[0281] Synthesis of Compound (No.122)
[0282]
[0283] Step 1:
[0284] (Methoxymethyl)triphenylphosphonium chloride (482.08g, 1.41mol) in THF (2L) was cooled to -60°C, potassium tert-butoxide (215.66g, 1.92mol) was added dropwise, and stirred for 1 Hour. Thereto, a THF (900 mL) solution of compound (T-3) (300.67 g, 1.26 mol) synthesized by a known method was added dropwise, and the mixture was returned to room temperature while stirring. The reaction mixture was poured into water, subjected to usual post-treatment, and purified by silica gel chromatography to obtain compound (T-4) (293.17 g, 1.10 mol; 87%).
[0285] Step 2:
[0286] Add 6N hydrochloric acid (180mL, 1.08mol) dropwise to compound (T-4) (293.17g, 1.10mol) and 2,2-dimethyl-1,3-propanediol (125.71g, 1.21mol) in acetone solution , and stirred at room temperature for several days. The usual post-treatment was carried out, followed by purification by silica gel chromatography and recrystallization to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com