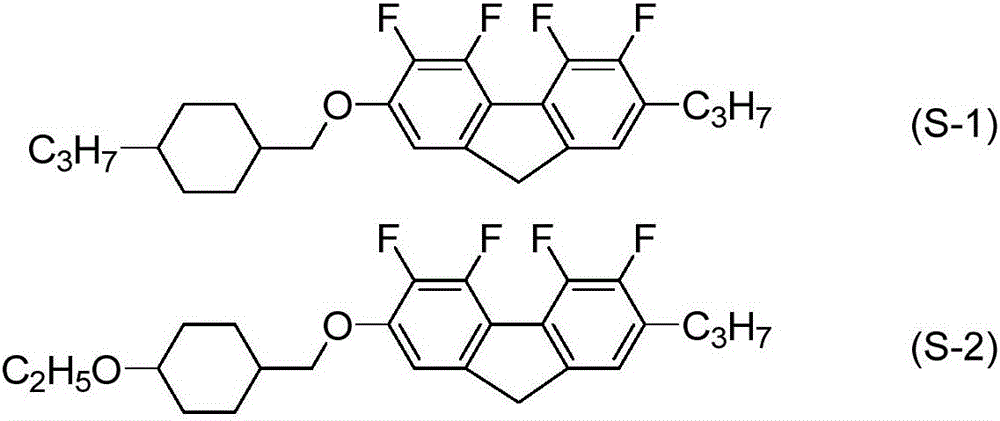
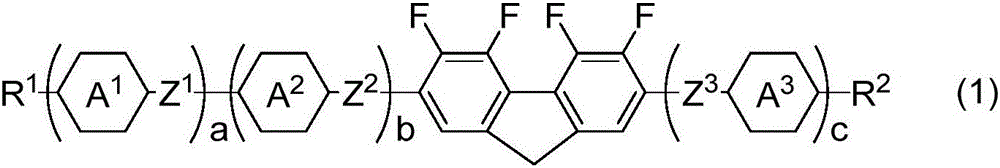
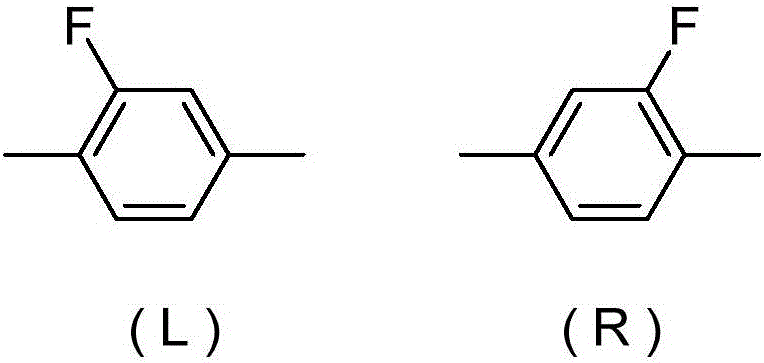
Liquid crystalline compound containing tetrafluorofluorene, liquid crystal composition, and liquid crystal display device
A liquid crystal composition and compound technology, applied in organic chemistry, liquid crystal materials, nonlinear optics, etc., can solve problems such as insufficient compatibility, high melting point, and large dielectric constant anisotropy, and achieve short response time, The effect of long life and high transparency point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0197] Composition (1) is prepared by a method such as dissolving essential components at high temperature. Additives can also be added to this composition according to the use. Examples of additives are optically active compounds, polymerizable compounds, polymerization initiators, antioxidants, ultraviolet absorbers, light stabilizers, heat stabilizers, defoamers, and the like. Such additives are widely known to those skilled in the art and are described in the literature.
[0198] Composition (1) may also further contain at least one optically active compound. Well-known chiral dopants can be added as optically active compounds. This chiral dopant has the effect of inducing a helical structure in liquid crystal molecules to impart a necessary twist angle, thereby preventing reverse twisting. Preferable examples of the chiral dopant include compound (Op-1) to compound (Op-18). In compound (Op-18), ring J is 1,4-cyclohexylene or 1,4-phenylene, R 24 is an alkyl group havi...
Embodiment 1
[0261] Synthesis of Compound (No.1-2-5)
[0262]
[0263] 1st process
[0264] Under nitrogen atmosphere, magnesium (20.8 g) and tetrahydrofuran (Tetrahydrofuran, THF) (30.0 ml) were put into the reactor and heated to 45° C. A THF (150 ml) solution of compound (T-1) (150 g) was slowly added thereto at a temperature range of 45°C to 55°C and stirred for 2 hours. Next, it cooled to 0 degreeC, the THF (200 ml) solution of compound (T-2) (127g) was added slowly, and it stirred for 2 hours, returning to room temperature. The reaction mixture was poured into saturated aqueous ammonium chloride, and the aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with water, and dried with anhydrous magnesium sulfate. The solution was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (volume ratio, toluene:ethyl acetate=10:1) to obtain Compound (T-3) (188 g; 95%).
[0265] 2nd process
[0266] Under a nitro...
Embodiment 2
[0286] Synthesis of Compound (No.1-2-6)
[0287]
[0288] 1st process
[0289] Under a nitrogen atmosphere, compound (T-12) (196 g) and THF (5000 ml) synthesized by the same method as in the seventh step of Example 1 were put into a reactor and cooled to -70°C. Thereto, sec-butyllithium (1.01M; cyclohexane, n-hexane solution; 1000 ml) was slowly added and stirred for 3 hours. Next, iron (III) chloride (92.1 g) was added, and it stirred for 12 hours, returning to room temperature. The reaction mixture was poured into 1N hydrochloric acid, and the aqueous layer was extracted with toluene. The combined organic layer was washed with brine, and dried with anhydrous magnesium sulfate. The solution was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (heptane). Furthermore, purification was performed by recrystallization from heptane to obtain compound (1-2-6) (25.2 g; 13%).
[0290] Chemical shift δ (ppm; CDCl 3 ):7.09(d,J=5.4Hz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com