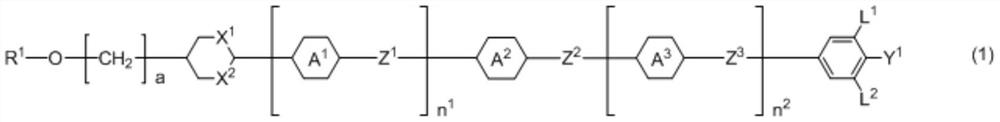
Liquid crystal composition, polymer/liquid crystal composite material, optical element, compound
A technology of liquid crystal composition and compound, which is applied in liquid crystal materials, optics, organic chemistry, etc., and can solve problems such as large dielectric anisotropy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0481] Composition (1) is prepared by a method such as dissolving desired components at high temperature. Depending on the application, additives may be added to the composition. Examples of additives are optically active compounds, polymerizable compounds, polymerization initiators, antioxidants, ultraviolet absorbers, light stabilizers, heat stabilizers, defoamers, pigments, and the like. Such additives are well known to those skilled in the art and are described in the literature.
[0482] Composition (1) may further contain at least one optically active compound. The optically active compound has an effect of preventing reverse twist by giving a desired twist angle (torsion angle) by generating a helical structure in the liquid crystal molecule. Preferable examples of the optically active compound include the following compound (K-1) to compound (K-16).
[0483]
[0484] Composition (1) adds such an optically active compound to adjust the helical pitch. In the case ...
Embodiment 1
[0647] 2-(4-(Difluoro-((2,3',4',5'-tetrafluoro-[1,1'-biphenyl]-4-yl)oxy)methyl)-3,5- Synthesis of Difluorophenyl)-5-(ethoxymethyl)-1,3-dioxane (No.148)
[0648]
[0649] The synthesis process is shown in the figure below.
[0650]
[0651] Step 1
[0652] Under nitrogen atmosphere, put triethyl methanetricarboxylate (0.90g, 8.5mmol), p-toluenesulfonic acid monohydrate (0.05g, 0.25mmol), acetone (27ml) into reaction vessel, stir at room temperature 12 hours. Triethylamine was added to the reaction mixture, and the solvent was distilled off with an evaporator. The residue was purified by silica gel chromatography to obtain compound (S102) (1.1 g, 7.8 mmol; 91%).
[0653] Step 2
[0654] Under nitrogen atmosphere, the compound (S102) (1.1g, 7.8mmol), sodium hydride (60%; 0.47g, 11.7mmol), and tetrahydrofuran (tetrahydrofuran, THF) (11ml) obtained in the first step were put into a reaction vessel , stirred at room temperature for 30 minutes. 1-Iodoethane (3.6 g, 23.4 ...
Embodiment 2
[0665] 5-(butoxymethyl)-2-(4-(difluoro-((2,3',4'-trifluoro-[1,1'-biphenyl]-4-yl)oxy)methyl Synthesis of -3,5-difluorophenyl)-1,3-dioxane (No.142)
[0666]
[0667] Compound (No. 142) was synthesized by a method similar to that of Example 1.
[0668] 1 H-NMR (δppm; CDCl 3 ):7.38-7.32(m,2H),7.25-7.10(m,2H),7.17-7.09(m,4H),5.39(s,1H),4.28(dd,2H,J=4.6Hz,11.8Hz) ,3.74(dd,2H,J=11.8Hz,11.8Hz),3.38(t,2H,J=6.6Hz),3.27(d,2H,J=5.8Hz),2.43(m,1H),1.57-1.50 (m,2H),1.36(dq,2H,J=7.5Hz,7.5Hz),0.93(t,3H,J=7.5Hz).
[0669] 19 F-NMR (δppm; CFCl 3 ):-61.40(t,2F,J=28.4Hz),-110.48(dt,2F,J=10.6Hz,28.4Hz),-115.14(dd,1F,J=8.5Hz,10.6Hz),-137.97- -138.07(m,1F),-139.25--139.35(m,1F).
[0670] The physical properties of the compound (No. 142) are as follows.
[0671] A mixture of the compound (15% by weight) and the mother liquid crystal (A) (85% by weight) was used as a sample. Based on these measured values, extrapolated values were calculated and described in accordance with the above-men...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric anisotropy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com