A Taqman real-time fluorescent PCR kit for detecting wild strains of porcine epidemic diarrhea virus in pig umbilical cord blood and its application
A porcine epidemic diarrhea, real-time fluorescence technology, applied in microorganism-based methods, microbial determination/inspection, microorganisms, etc., can solve the problems of affecting the amplification effect, high qPCR sensitivity, low sensitivity, etc., to achieve broad and specific. The effect of strong performance and reliable technical support
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
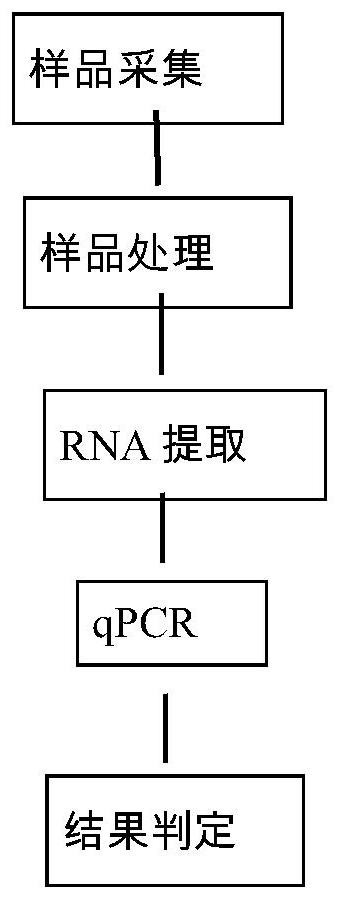
[0066] The flow chart of the present invention's detection of porcine epidemic diarrhea virus field strain in pig umbilical cord blood is as follows figure 1 shown. Specifically include the following steps: (1) sample collection; (2) sample processing; (3) RNA extraction; (4) qPCR: use the specific primers and probes designed in the present invention to carry out qPCR reaction; (5) determine the result.
[0067] 1. Sample collection
[0068] (1) Cord blood sample collection
[0069] a. Take a clean penicillin bottle and cork, clean it, boil and sterilize it for 30 minutes, dry it and collect it for later use;
[0070] b. When the piglets are born, squeeze the "cord blood" of all piglets delivered by each sow into a clean penicillin bottle, 3-5 drops per piglet, and squeeze the "cord blood" into penicillin bottle, sealed;
[0071] Precautions: ①It is necessary to collect all the piglets from the same litter sow to avoid missed detection caused by individual differences in t...
Embodiment 2
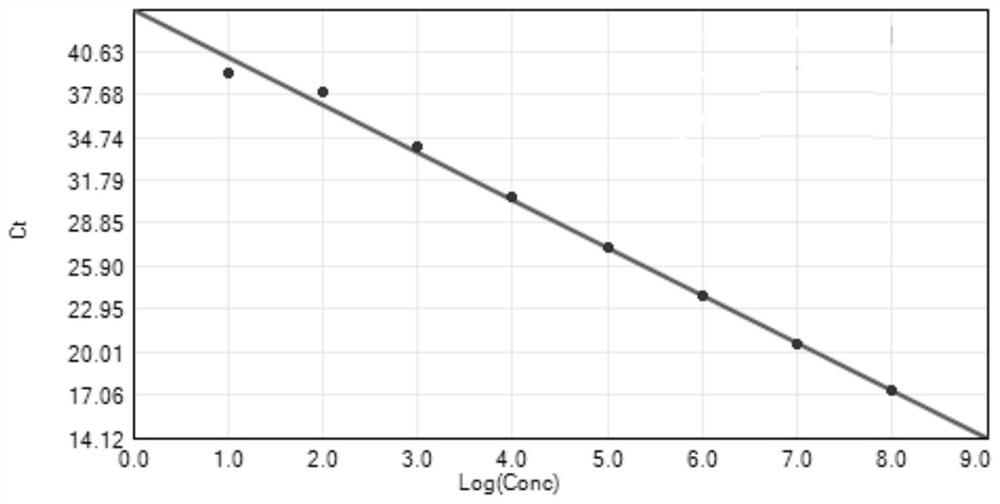
[0134] Embodiment 2 Sensitivity research
[0135] Evaluate the sensitivity of the kit of the present invention with positive control cloning plasmid, carry out 10 times gradient dilutions to cloning plasmid, detection range is 10 8 -10 1 copies / μl. The results show that the detection range of the method is 10 8 -10 1 Copies / μl, reliable results can be obtained for porcine epidemic diarrhea virus content in this range, that is, the sensitivity of this method can detect samples with porcine epidemic diarrhea virus content of 10 copies. See the test results Figure 5 .
Embodiment 3
[0136] Embodiment 3 specificity research
[0137] In order to detect the specificity of the kit of the present invention, utilize the kit of the present invention to detect blue ear classical strain, porcine parvovirus, porcine rotavirus, porcine transmissible gastroenteritis virus, porcine circovirus, porcine pseudorabies virus, B Type encephalitis, swine fever virus and other 8 kinds of viruses.
[0138] The test results show that the kit of the invention can only amplify the porcine epidemic diarrhea virus in the umbilical cord blood of piglets, indicating that the kit of the invention can specifically amplify the porcine epidemic diarrhea virus without cross-reacting with other nucleic acids. See the test results Figure 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com