Primer for PCR (polymerase chain reaction) diagnosis of wild porcine parvovirus infection, kit with primer for PCR diagnosis of wild porcine parvovirus infection and application of primer and kit
A parvovirus and kit technology, applied in the field of primers for PCR diagnosis of porcine parvovirus wild virus infection, can solve the problems of being unsuitable for large-scale clinical diagnosis and field application, producing non-target bands, and low specificity of primers. Simple result judgment, short detection time and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Extraction of DNA from Umbilical Cord Blood
[0046] (1) Collection of umbilical cord blood
[0047] Cord blood collection includes the following steps: a. Take a clean penicillin bottle and bottle stopper, clean them, boil and sterilize them for 30 minutes, dry them and collect them for later use;
[0048] b. When the piglets are born, squeeze the "cord blood" of all piglets delivered by each sow into a clean penicillin bottle, 3-5 drops per piglet, and squeeze the "cord blood" into penicillin bottle, sealed;
[0049] Precautions: ①It is necessary to collect all the piglets from the same litter sow to avoid missed detection caused by individual differences in the piglets from the same litter sow; ②It can be operated by two people or alone. You can cut the umbilical cord into several sections and then operate it; ③If the sow is mummified or stillborn, the umbilical cord blood of the piglets in the same litter should be tested;
[0050] c. After the umbilical cord ...
Embodiment 2
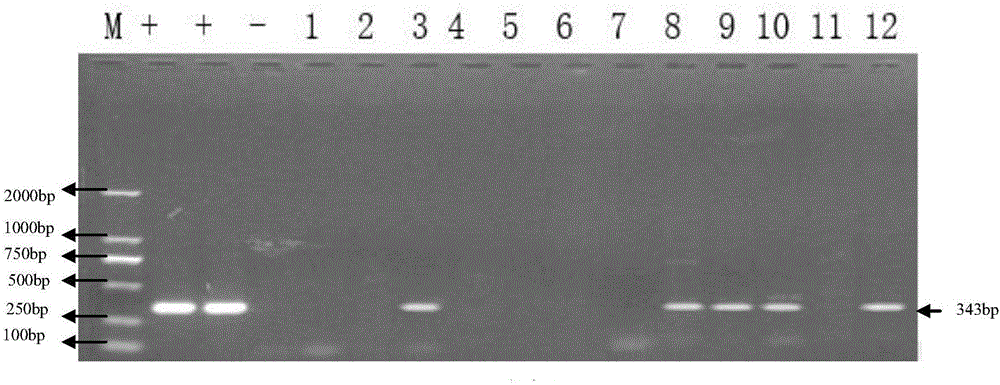
[0085] In a farm with 1,000 sows in the north, the sows were vaccinated with the parvovirus vaccine. In order to further evaluate the protective effect of the parvovirus vaccine on the sows and the infection of the piglets, the umbilical cord blood of piglets from 12 litters of farrowing sows was collected. According to the method described in Implementation 1, distinguish porcine parvovirus vaccine immunity and viral wild virus infection, evaluate the immune effect of porcine parvovirus vaccine, and the situation of piglets infected with porcine parvovirus. The result is as figure 1 shown.
[0086] From figure 1It can be seen that there is porcine parvovirus in the umbilical cord blood of the positive control sample, no porcine parvovirus is detected in the umbilical cord blood of the negative control sample, and VP2 of porcine parvovirus is present in the umbilical cord blood of samples 3, 8-10 and 12 The fragments in the gene further indicate the presence of porcine parvo...
Embodiment 3
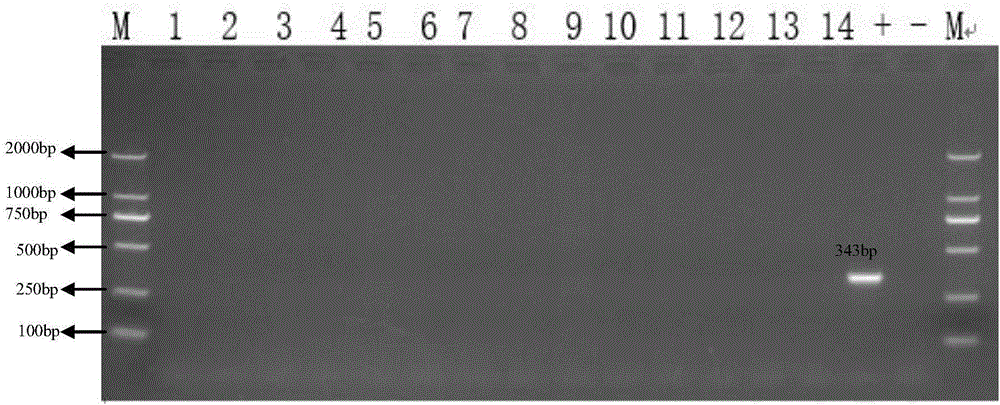
[0088] In a farm with 500 sows in the north, the sows were vaccinated with the parvovirus vaccine. In order to further evaluate the protective effect of the parvovirus vaccine on the sows and the infection of the piglets, the umbilical cord blood of piglets from 14 litters of farrowing sows was collected. According to the method described in Implementation 1, distinguish porcine parvovirus vaccine immunity and viral wild virus infection, evaluate the immune effect of porcine parvovirus vaccine, and the situation of piglets infected with porcine parvovirus. The result is as figure 2 shown.
[0089] From figure 2 It can be seen that there is porcine parvovirus in the umbilical cord blood of the positive control sample, porcine parvovirus is not detected in the umbilical cord blood of the negative control sample, and there is no porcine parvovirus in the VP2 gene of the umbilical cord blood of samples 1-14. Fragments, further indicating that there is no porcine parvovirus in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com