The drug composition of erlotinib
A technology of ibrutinib and composition, applied in the field of pharmaceutical composition of antitumor drug ibrutinib and CYP3A enzyme inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
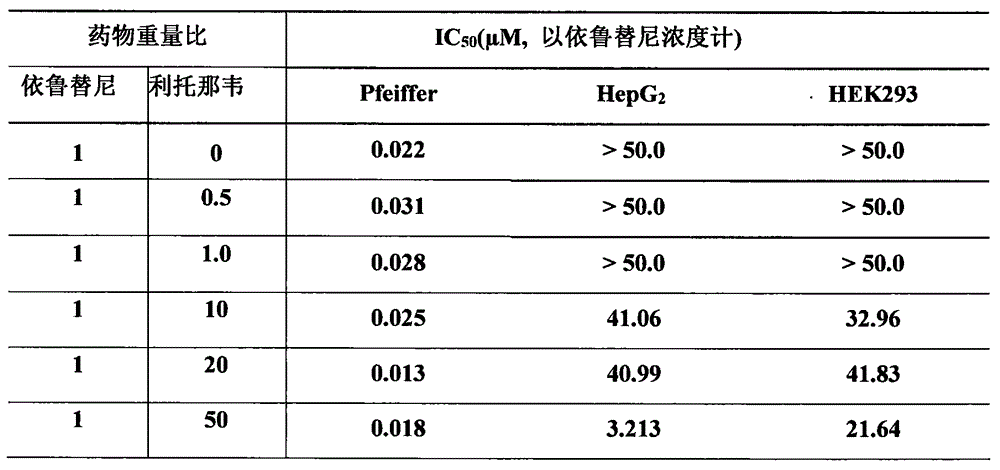
[0021] Example 1 The inhibitory effect of the composition of ibrutinib and ritonavir on target cells and non-target cells
[0022] The composition of different ratios of ibrutinib and ritonavir was tested by MTT method on Pfeiffer cells, purchased from Shanghai Enzyme Research Biotechnology Co., Ltd.), and non-target cells HepG2 cells and HEK293 cells (purchased from Shanghai Enzyme Research Biotechnology Co., Ltd.) inhibition.
[0023] Culture the cells to be tested, and prepare a concentration of about 2.5×10 4 cells / mL of cell suspension, seeded in 96-well culture plate, 100.0uL / well, placed at 37°C, 5% CO 2 Incubate in an incubator for 24 hours; add different concentrations of test substances to the 96-well plate of cultured tumor cells, and continue to culture for 72 hours; discard the culture medium, add 100.0 μL of 0.05% MTT application solution to each well, and incubate for 4 hours Discard the culture medium, add 100 μL DMSO to each well, shake for 5 minutes to diss...
Embodiment 2
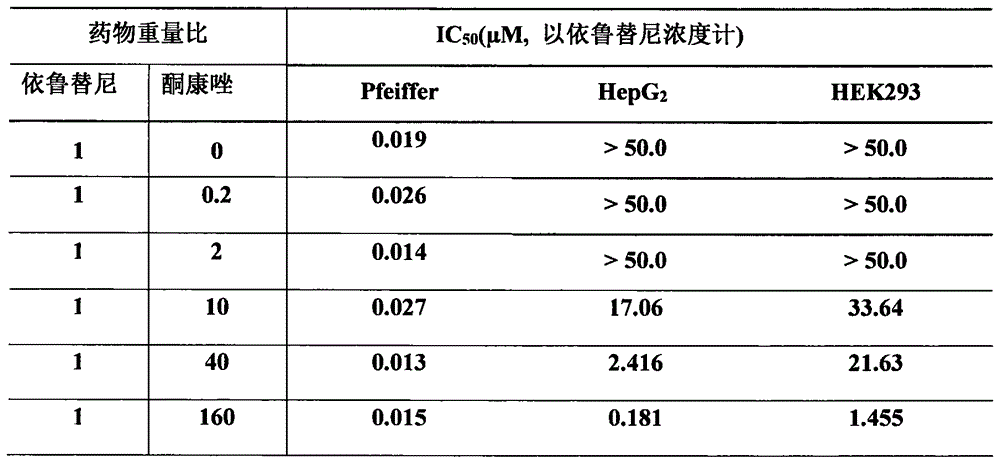
[0032] Example 2 The inhibitory effect of the composition of ibrutinib and ketoconazole on target cells and non-target cells
[0033] Referring to the method of Example 1, the inhibitory effect of the composition of ibrutinib and ketoconazole in different ratios on Pfeiffer cells, HepG2 cells and HEK293 cells was tested. The results are shown in Table 2.
[0034] Table 2 Ibrutinib and ketoconazole composition anti-tumor activity evaluation results
[0035]
[0036] It can be seen from Table 2 that the addition of different proportions of ketoconazole does not affect the inhibitory effect of ibrutinib on target cell lymphoma B lymphocytes; but the addition of a high proportion of ketoconazole increases the effect on non-target cells HepG2 cells ( liver-derived) and HEK293 cells (nephrogenic), especially HepG2 cells have significant toxicity, suggesting that the composition containing a high proportion of ketoconazole may have stronger liver toxicity.
Embodiment 3
[0037] Example 3 Inhibition of the Composition of Ibrutinib and Cobicistat on Target Cells and Non-Target Cells
[0038] Referring to the method of Example 1, the inhibitory effect of the composition of ibrutinib and cobicistat in different ratios on Pfeiffer cells, HepG2 cells and HEK293 cells was tested. The results are shown in Table 3.
[0039] Table 3 Evaluation results of the antitumor activity of the combination of ibrutinib and cobicistat
[0040]
[0041] It can be seen from Table 3 that the addition of different proportions of cobicistat does not affect the inhibitory effect of ibrutinib on target cell lymphoma B lymphocytes; but the addition of a high proportion of cobicistat increases the effect on non-target cells HepG2 cells ( liver-derived) and HEK293 cells (kidney-derived), suggesting that the composition containing a high proportion of cobicistat may have certain liver and kidney toxicity.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap