A compound capable of inhibiting IDO, its preparation method and its use
一种化合物、用途的技术,应用在医药领域,能够解决未找到很好IDO抑制剂等问题,达到强抑制活性、好应用前景的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
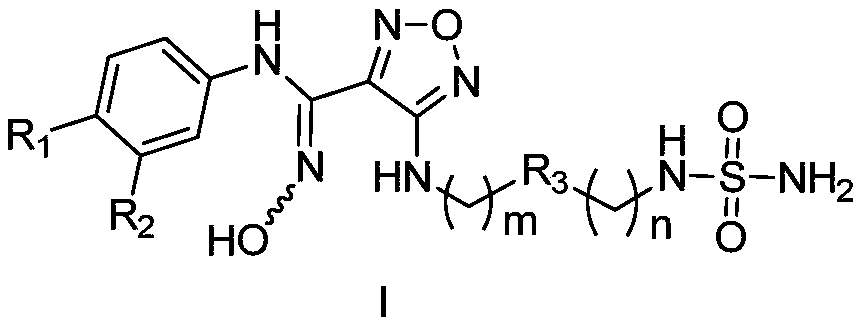
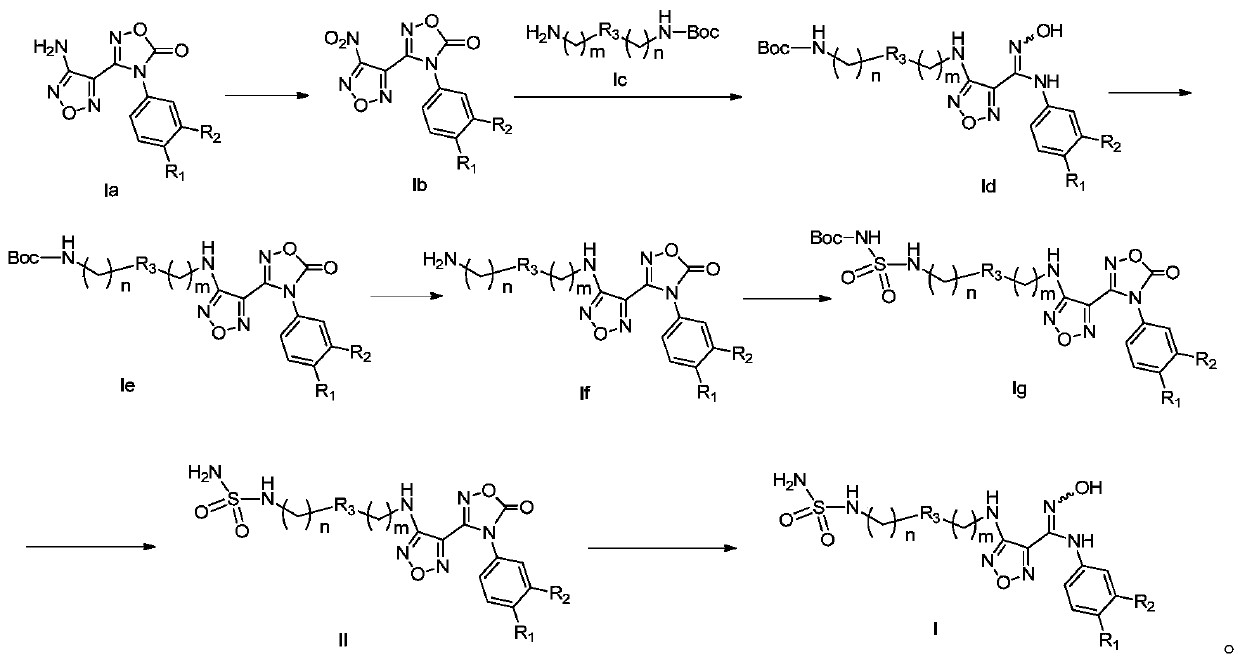
[0031] The preparation method of the compound represented by general formula (I) of the present invention or its salt, comprises the following steps:
[0032]
[0033]Under acidic conditions, the compound of general formula (Ia) is oxidized to the compound of general formula (Ib); the compound of general formula (Ib) reacts with the compound of general formula (Ic) under alkaline conditions to obtain the compound of general formula (Id); The compound of formula (Id) forms a ring under heating and basic conditions, and the base under this condition is preferably N, N'-carbonyldiimidazole to obtain a compound of general formula (Ie); the compound of general formula (Ie) after ring formation is Remove the protecting group on the amino under the condition, obtain general formula (If) compound or its salt; ) compound, the preferred tert-butanol solution of the alcoholic solution; the compound of general formula (Ig) removes the protecting group on the amino group under acidic co...
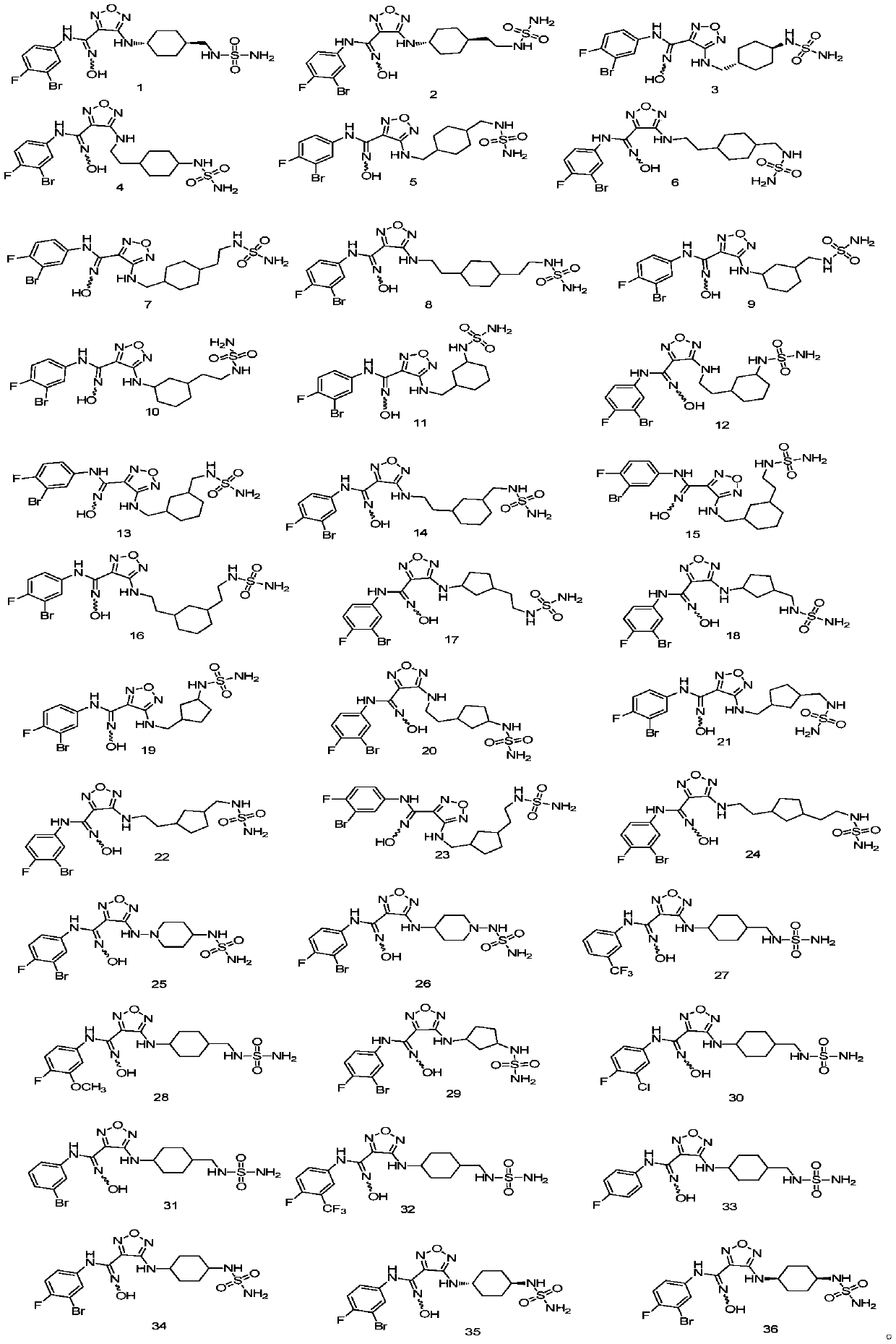
Embodiment 1
[0046] trans-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((4-((sulfamoylamino)methyl)cyclohexyl)amino)-1,2,5-oxadiazole-3-carboximidamide, the compound The preparation of 1, wherein the structural formula of compound 1 is as follows:
[0047]
[0048] The first step, prepare compound lb:
[0049] The raw material compound la (500mg, 1.46mmol, prepared by referring to the method disclosed in P53 Example3 stepA in the patent application "WO2010005958") was added to 7mL of trifluoroacetic acid, then 6mL of hydrogen peroxide (30%) was added, and the reaction was carried out at 45°C for 20 hours . After the reaction, quench the reaction by adding saturated sodium sulfite solution, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure, and purify the resulting residue by silica gel column chromatography with eluent system B Compound lb (410 mg, yellow oil) was obtained with a yield of 7...
Embodiment 2
[0065] trans-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((4-(2-(sulfamoylamino)ethyl)cyclohexyl)amino)-1,2,5-oxadiazole-3-carboximidamide, That is the preparation of compound 2, wherein the structural formula of compound 2 is as follows:
[0066]
[0067] Using the synthetic method of Example 1, the second step raw material compound 1c is replaced by (commercially available) to obtain compound 2 (73 mg, white solid), with a yield of 45%. MS m / z(ESI):521.4[M+1] + , 1 HNMR(400MHz,DMSO-d6)δ11.52(s,1H),8.87(s,1H),7.09-7.27(m,3H),6.76-6.79(m,1H),6.67(s,2H),6.21 (t,1H), 2.41(m,1H), 1.20-1.83(m,11H), 3.55(d,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com