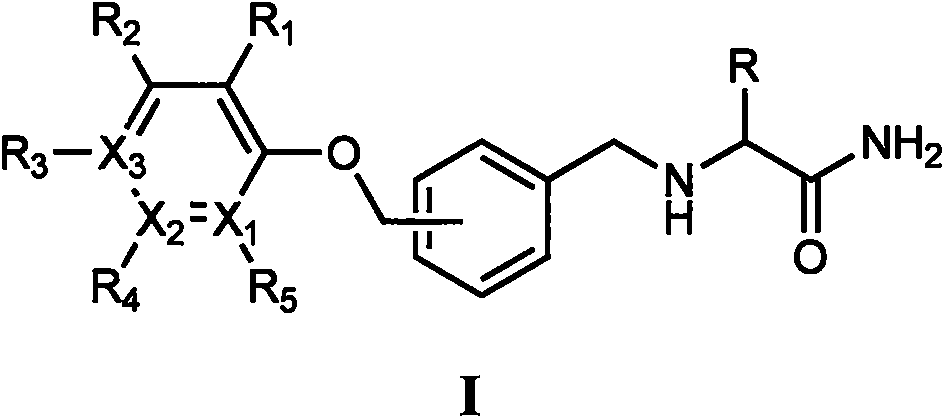
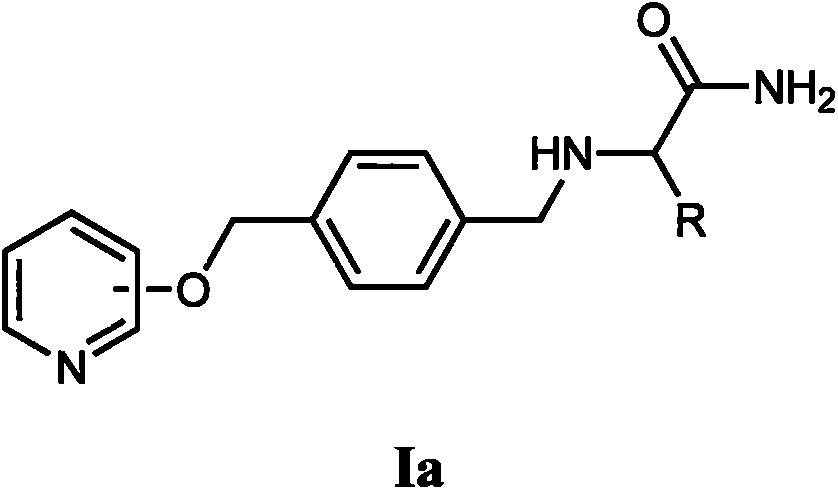
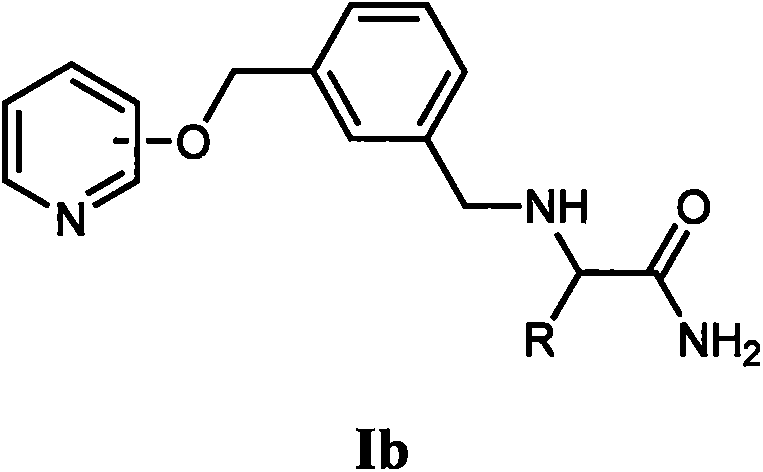
Novel alpha-amino amide derivatives and pharmaceutical use thereof
A pharmacy and compound technology, applied in the field of new α-aminoamide derivatives and their medical applications, can solve the problem that the analgesic activity of Ralfinamide needs to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 (2S)-2-(4-(pyridine-2-oxymethyl) benzyl) amino-propionamide (Ia-1) synthesis
[0044]
[0045] 1.1 Synthesis of 4-hydroxymethylbenzaldehyde (II-1)
[0046] Take a 500mL eggplant-shaped bottle, add 20g of terephthalaldehyde (0.15mol; 4.0equiv), 100ml of ethanol and 150ml of tetrahydrofuran into the bottle in turn, stir and dissolve evenly. Then, under ice-bath conditions, slowly add 1.7g of sodium borohydride solid (9.3mmol; 1.0equiv) to the bottle at one time, react for more than 6h, spot the plate with thin-layer TLC, and monitor the reaction progress with an ultraviolet analyzer (254nm). After the raw material point of terephthalaldehyde completely disappears, stop the reaction, add dropwise the 2mol / L hydrochloric acid solution prepared in advance to quench, adjust the pH value to 4-5, and then rotate the reaction solution to dryness, and the obtained residue is water, acetic acid Ethyl ester was redissolved and added to a separatory funnel. The aqu...
Embodiment 2
[0053] Synthesis of Example 2 (2S)-2-(4-(pyridine-3-oxymethyl)benzyl)amino-propionamide (Ia-2)
[0054]
[0055] 2.1 Preparation of 4-(pyridine-3-oxymethyl)-benzaldehyde (IV-1b)
[0056] Take a 250mL eggplant-shaped bottle, weigh 1.0g intermediate III-1 (5.0mmol; 1.0equiv), 0.48g 3-hydroxypyridine (5.0mmol; 1.0equiv), 2.1g potassium carbonate (15.0mmol; 3.0equiv) , 0.6g of potassium iodide (3.0mmol; 0.6equiv), and 60mL of acetone were added to the bottle, stirred evenly, then heated to 58°C for reflux reaction for 24h, spotted by thin-layer TLC, and monitored by an ultraviolet analyzer (254nm). Then the reaction solution was filtered and evaporated to dryness, and the resulting residue was redissolved with 2mol / L NaOH aqueous solution and ethyl acetate and added to a separatory funnel. The aqueous phase was extracted 2 to 3 times with an equal volume of ethyl acetate, the ethyl acetate layers were combined, washed with saturated aqueous sodium chloride solution, and then t...
Embodiment 3
[0059] The synthesis of embodiment 3 (2S)-2-(4-(pyridine-3-oxymethyl) benzyl) amino-propionamide (Ia-3)
[0060]
[0061] 3.1 Preparation of 4-(pyridine-4-oxymethyl)-benzaldehyde (IV-1c)
[0062] Take a 250mL eggplant-shaped bottle, weigh 1.0g intermediate III-1 (5.0mmol; 1.0equiv), 0.48g 4-hydroxypyridine (5.0mmol; 1.0equiv), 2.1g potassium carbonate (15.0mmol; 3.0equiv) , 0.6g of potassium iodide (3.0mmol; 0.6equiv), and 60mL of acetone were added to the bottle, stirred evenly, then heated to 58°C for reflux reaction for 24h, spotted by thin-layer TLC, and monitored by an ultraviolet analyzer (254nm). Then the reaction solution was filtered and evaporated to dryness, and the resulting residue was redissolved with 2mol / L NaOH aqueous solution and ethyl acetate and added to a separatory funnel. The aqueous phase was extracted 2 to 3 times with an equal volume of ethyl acetate, the ethyl acetate layers were combined, washed with saturated aqueous sodium chloride solution, a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap