Fluorescent quantitative PCR kit for human spinal muscular atrophy-related gene deletion detection
A technology for spinal muscular atrophy and gene deletion, which is used in the determination/examination of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc. It can solve the problems of homozygous deletion and heterozygous mutation, and achieve the effect of avoiding interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] In order to make the object, technical solution and advantages of the present invention clearer, the following will be further described in detail in conjunction with the embodiments. The specific embodiments described here are only used to explain the present invention, not to limit the present invention. In the following examples, DNA polymerase was purchased from Takara Company (PCR buffer, dNTP, MgCl 2 All provided together with the enzyme), the primers, probes and plasmids used were synthesized by Suzhou Synbio Biotechnology Co., Ltd., and the blood genomic DNA extraction kit was purchased from Tiangen Biochemical Technology Co., Ltd.
[0052] Sample DNA was prepared using the Blood Genomic DNA Extraction Kit in the following examples.
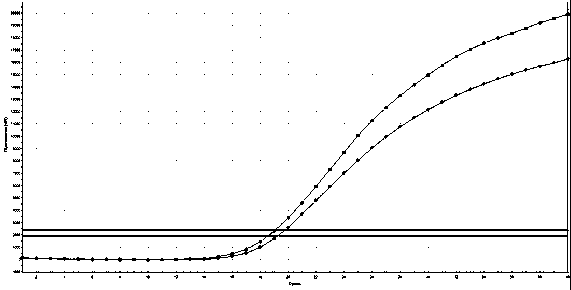
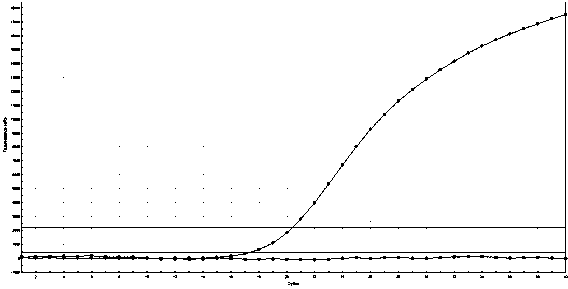
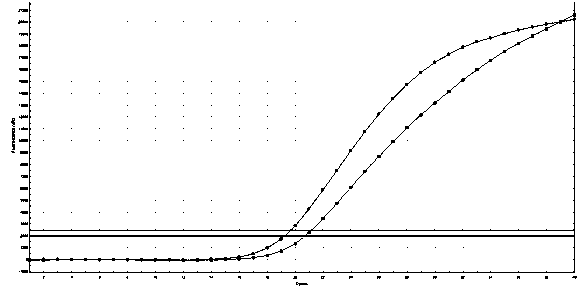
[0053] In Example 1, on the basis of conventional PCR primer design, the present invention artificially sets a mismatched base, which enhances the specificity of the primer and effectively prevents non-specific amplification.
[...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap