A kind of IgG1 Fc monomer and its application
A monomer and application technology, applied in the field of its preparation, IgG1Fc monomer, can solve problems such as limiting clinical use space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
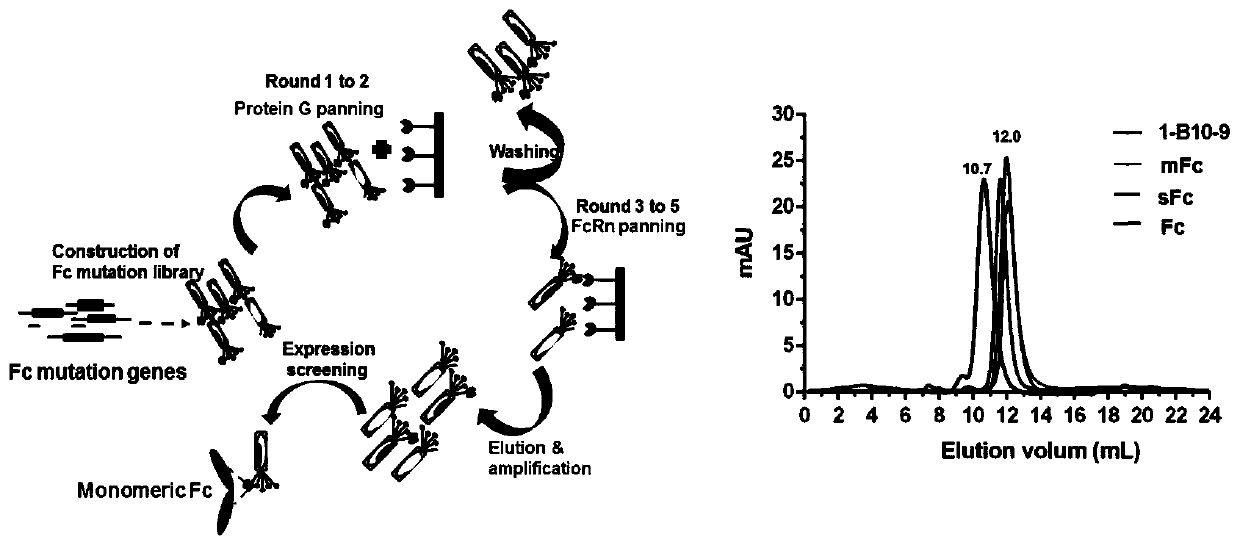
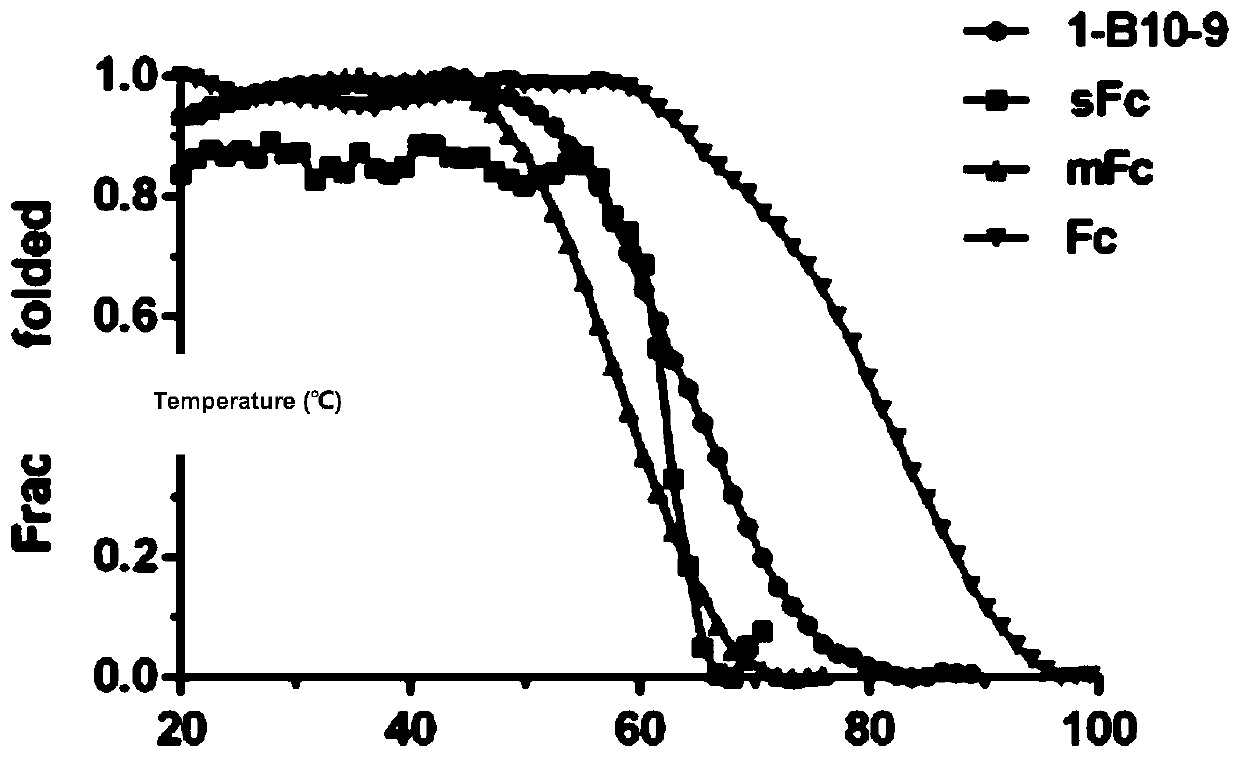
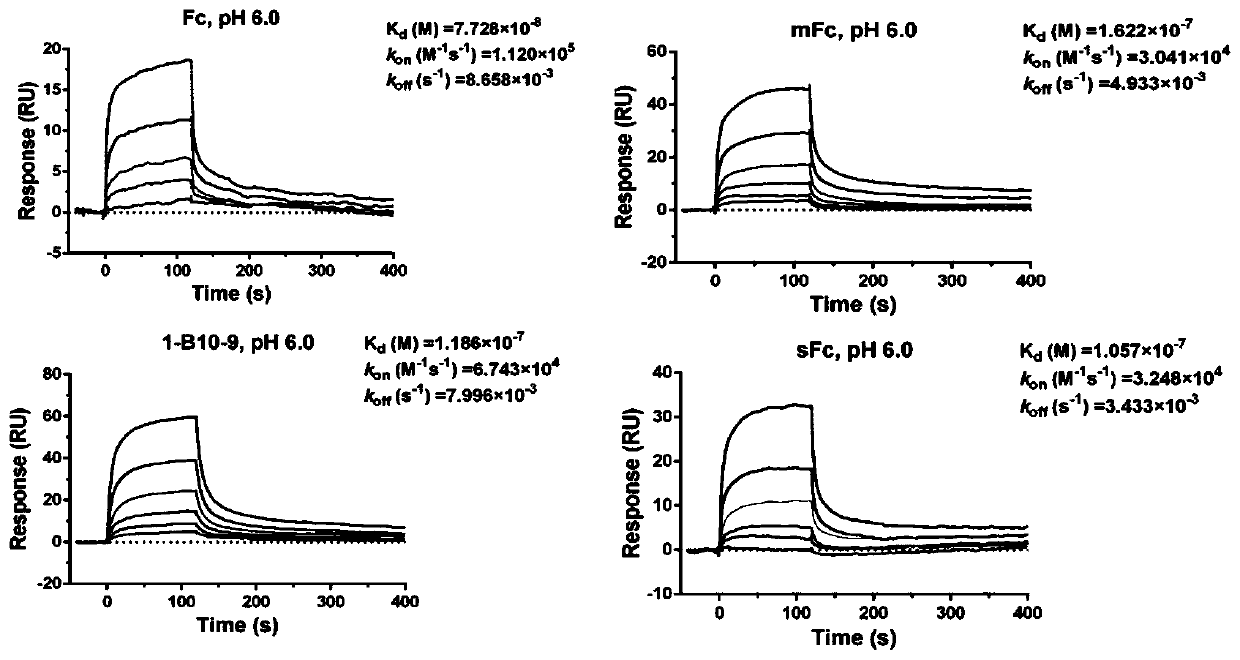
[0063] Example 1 Screening and determination of IgG1 Fc monomer (sFc)
[0064] Mutations were carried out in the IgG1 constant region, including four site-directed mutation sites and one random mutation site (Leu-351, Thr-366, Leu-368, Pro-395, Lys-409) related to monomer formation, and There are two random mutation sites (Met-428, Asn-434) related to FcRn binding, based on which an IgG1 Fc mutant antibody library with a library capacity of 1.28×10 5 was constructed. Screening of novel IgG1 Fc monomers against biotin-labeled soluble FcRn proteins; biotin-labeled soluble FcRn was immobilized on streptavidin-coated magnetic beads, and 10-12 phage-displayed Fc were incubated at room temperature at 1, 2 rounds of incubation with proteinG; 3, 4, and 5 rounds of incubation with 5, 4, and 2 micrograms of FcRn antigen for two hours respectively. Each round of screening used 10 12 phages, and polyclonal phage ELISA was used to detect the enrichment of antibodies. In the 3rd, 4th, and ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap