Use of cyclo tyrosine-valine and analog compounds to treat asthma and airway allergy
A technology of tyrosine and valine, applied in allergic diseases, respiratory diseases, drug combinations, etc., can solve problems such as adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
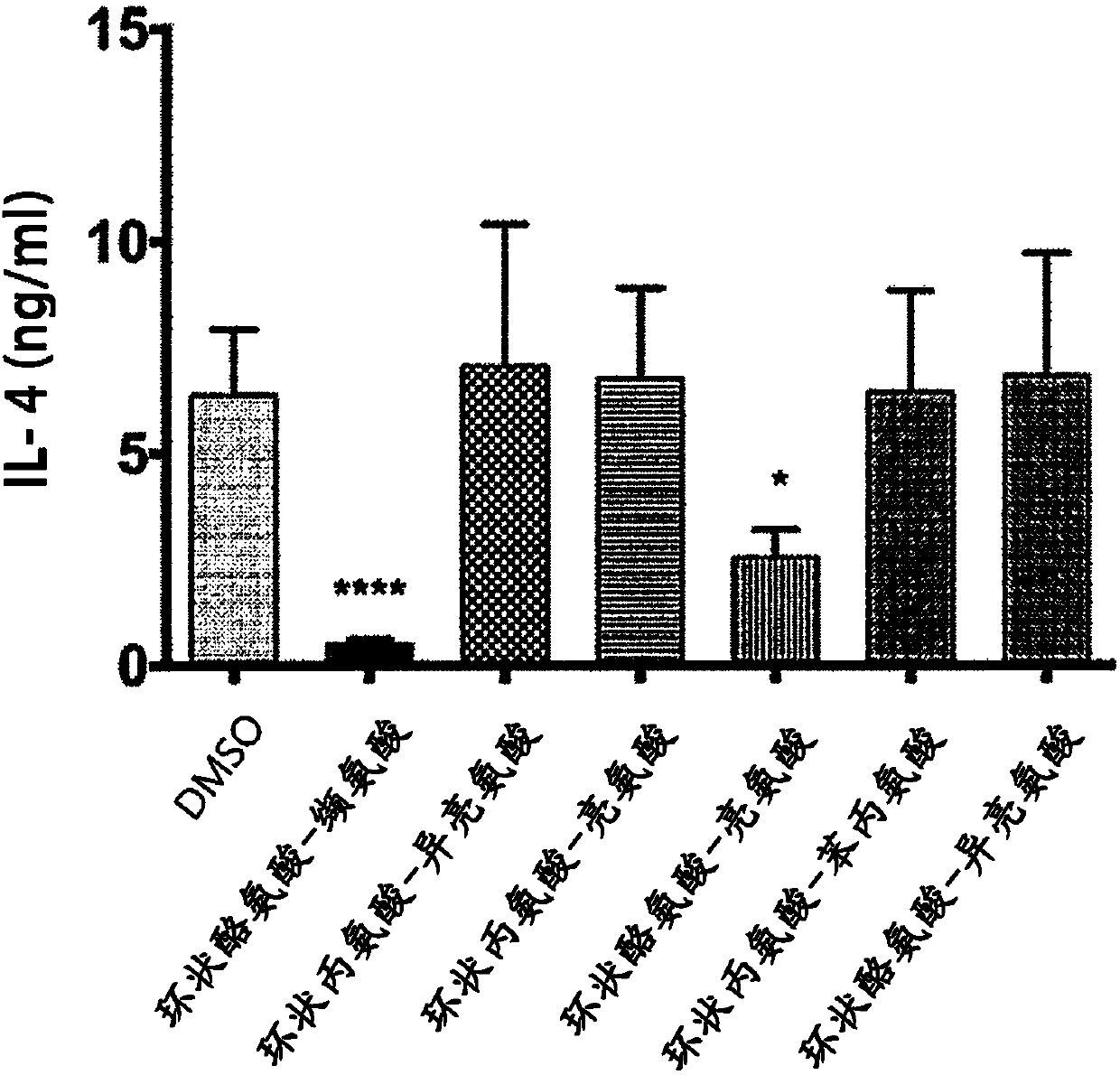
[0035] Cyclic tyrosine-valine inhibits Th2 cells from secreting inflammatory precursor hormones
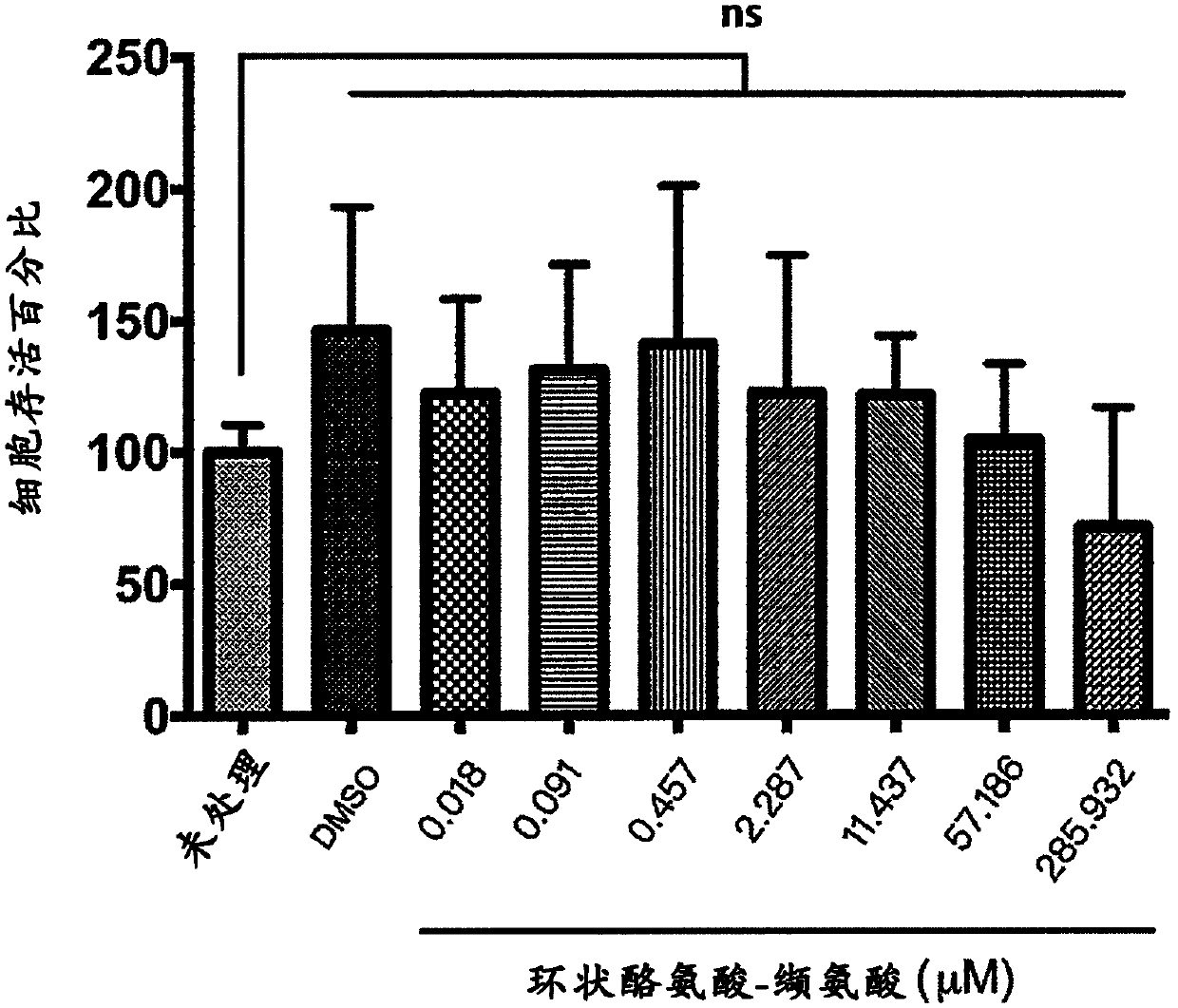
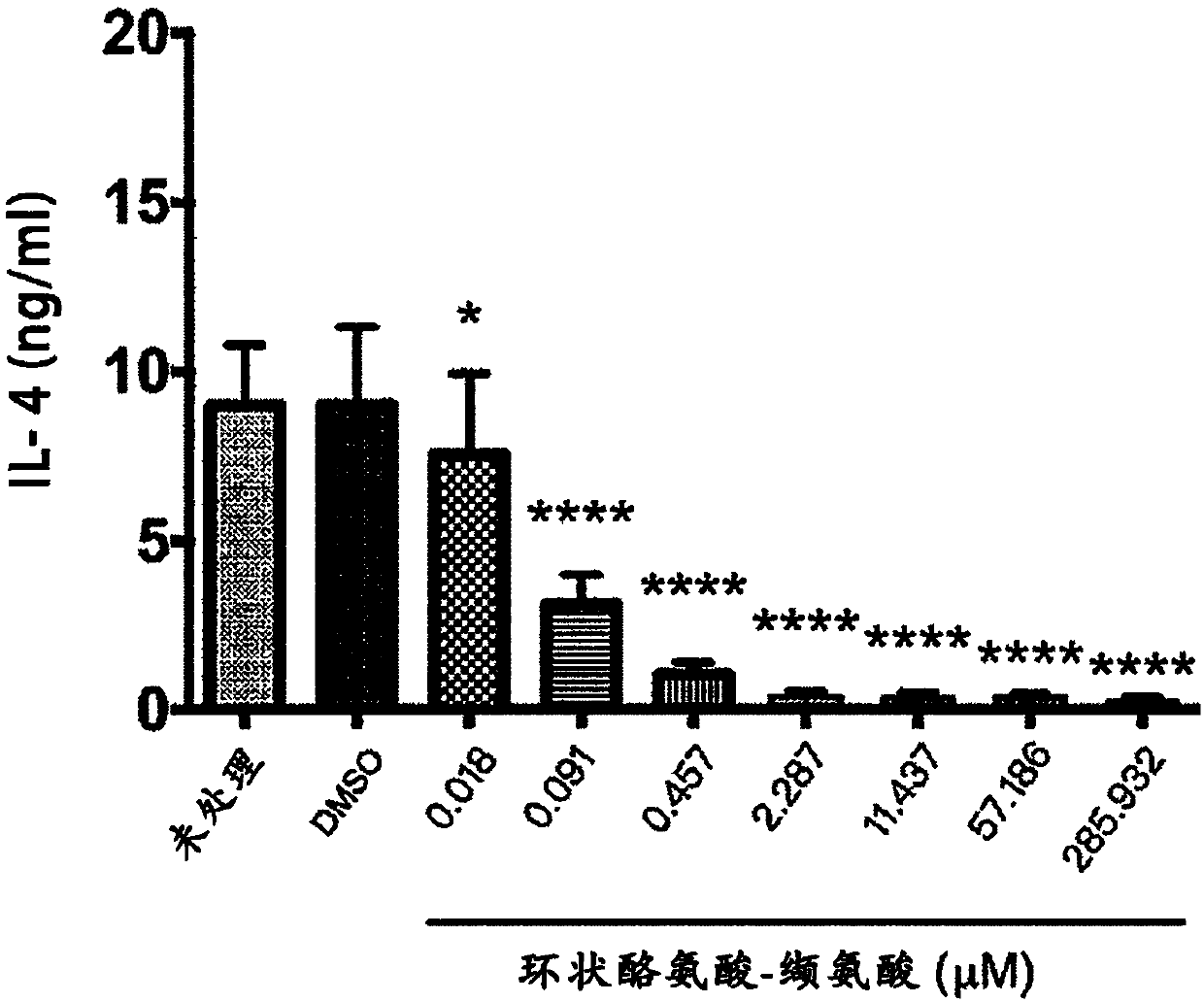
[0036] In view of the immunological effects of Th2 cells regulating asthma in humans and animals, the effect of cyclic tyrosine-valine on Th2 cells in culture was tested in this example. First, naive T cells were isolated from BALB / c mice cultured on a culture medium containing anti-CD3 antibody (2 μg / ml), anti-CD28 antibody (2 μg / ml), anti-interferon-γ (IFN-γ) antibody (10 μg / ml), and IL-4 (10ng / ml) medium to induce T cells to differentiate into Th2 cells. The Th2 cells were treated with 2.3 μM cyclic tyrosine-valine (Cyclo Tyr-Val for short), cyclic alanine-isoleucine (Cyclo Ala-Ile), cyclic alanine-leucine (Cyclo Ala-Leu), cyclic tyrosine-leucine (Cyclo Tyr-Leu), cyclic alanine-phenylalanine (Cyclo Ala-Phe for short), or cyclic tyrosine-isoleucine Tyr-Ile (Cyclo Tyr-Ile) was treated for three days, and then the protein content of IL-4 was determined by enzyme-linked immunoso...
Embodiment 2
[0041] Cyclic tyrosine-valine inhibits airway hyperresponse (AHR) in mice
[0042] In view of the fact that one of the characteristics of asthma is the excessive response of the respiratory tract to irritants, this embodiment takes ovalbumin as an example of an allergen, and tests the effect of cyclic tyrosine-valine on stimulation by methacholine (Mch). Effects of Ovalbumin-Induced Airway Hyperresponse in Asthmatic Mice. First, BALB / c mice were given 50 μg of ovalbumin (Sigma, USA) and 0.8 mg of aluminum hydroxide (Thermo Fischer Scientific, USA) by intraperitoneal injection (intraperitoneal injection) on days 0, 1, 2, and 14. mixture, and on days 14, 17, 21, 24, and 27, the mice were given 2% ovalbumin with a nebulizer (Plumo-Aide 5650; Devilbiss, USA) for 20 minutes. Thereafter, on days 21 to 27, the vehicle (normal saline containing 0.5% DMSO) or cyclic tyrosine-valine at a dose of 0.11304, 1.1304, 11.304, or 113.04 nmol / kg body weight was injected intraperitoneally. The...
Embodiment 3
[0044] Cyclic tyrosine-valine reduces cellular infiltration, proinflammatory hormone secretion, and antibody production in mouse lungs
[0045] In view of the fact that one of the characteristics of asthma is the accumulation of immune cells including eosinophilic leukocytes in the lung tissue, this example detects the effect of cyclic tyrosine-valine on the number of cells in the lung washing fluid, and at the same time explores its effect on inflammation Effects of precursor hormone secretion and antibody production. First, ovalbumin-induced asthmatic mice (BALB / c mice) were established according to the method in Example 2. Vehicle (normal saline containing 0.5% DMSO) or cyclic tyrosine-valine at doses of 0.11304, 1.1304, 11.304, or 113.04 nmol / kg body weight were administered intraperitoneally daily on days 21 to 27 The ovalbumin-induced asthmatic mice were collected, and the lung washing fluid and serum of the mice or mice treated with saline (control group) were collecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com