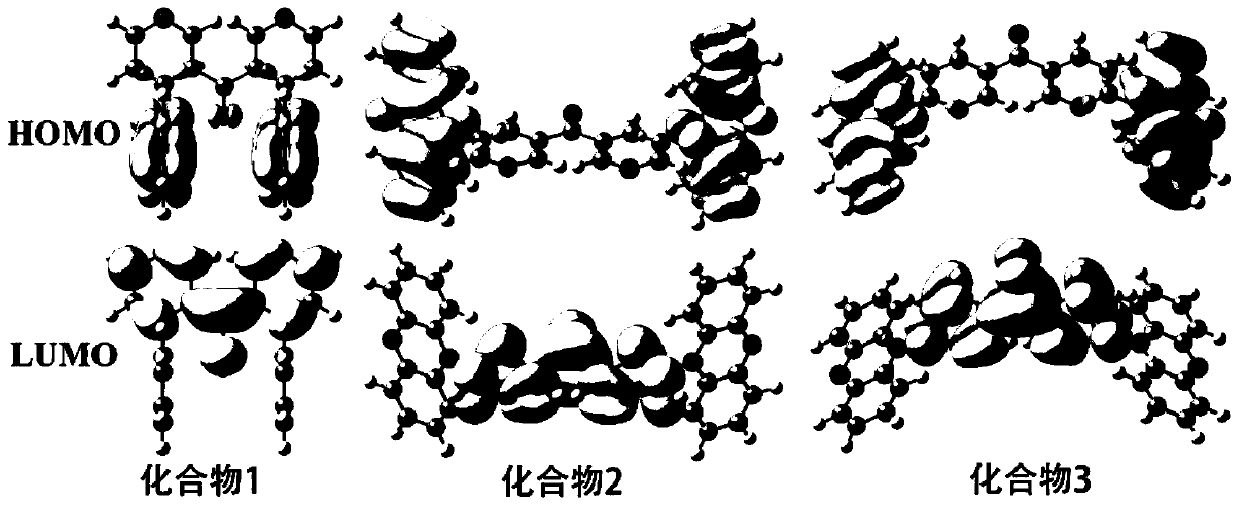
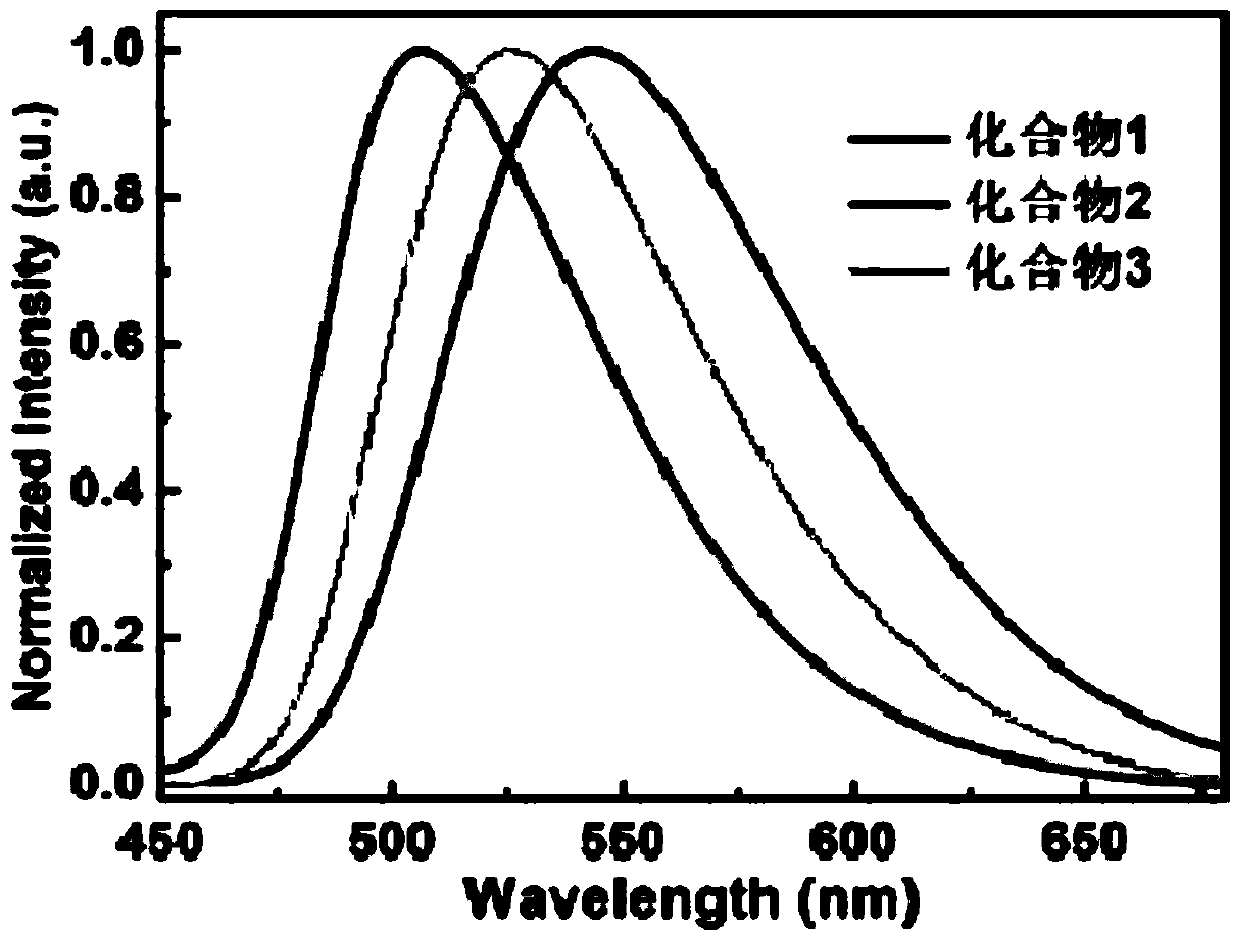
Thermally-activated delayed fluorescent material, preparation method thereof, and organic light-emitting diode device
A technology for heat-activated delayed and fluorescent materials, applied in luminescent materials, electrical solid devices, chemical instruments and methods, etc., can solve problems such as the lack of heavy metal Ir complexes, achieve low singlet triplet energy level difference, improve luminous efficiency, and high Effect of Device Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The synthetic route of target compound 1 is as follows:
[0044]
[0045] Add raw material 1 (2.09g, 5mmol) in 100mL two-necked flask, phenoxazine (2.2g, 12mmol), palladium acetate Pb (OAc) (90mg, 0.4mmol) and tri-tert-butylphosphine tetrafluoroborate ( t-Bu) 3 HPBF 4 (0.34g, 1.2mmol), then sodium tert-butoxide NaOt-Bu (1.16g, 12mmol) was added in the glove box, and 60mL of toluene that had been dehydrated and deoxygenated was injected under an argon atmosphere, and reacted at 120°C for 24 hours . Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 3:2) to obtain 1.8 g of compound 1 as grass-green powder, yield 66%.
[0046] 1HNMR (300MHz, CD 2 Cl 2 ,δ): 8.73 (s, 2H), 8.36 (d, J=6.3Hz, 2H), 7.14-6.90 (m, 18H).
[0047] MS(EI)m / z:[M] + calcd for C 35 h 22 N 4 o 3 ...
Embodiment 2
[0049] The synthetic route of target compound 2 is as follows:
[0050]
[0051] Add raw material 2 (2.09g, 5mmol), phenoxazine (2.2g, 12mmol), palladium acetate (90mg, 0.4mmol) and tri-tert-butylphosphine tetrafluoroborate (0.34g, 1.2mmol) in 100mL two-necked flask mmol), then sodium tert-butoxide (1.16 g, 12 mmol) was added into the glove box, and 60 mL of toluene previously dehydrated and deoxygenated was poured into the glove box under an argon atmosphere, and reacted at 120° C. for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 3:2) to obtain 1.6 g of compound 2 as grass-green powder, yield 59%.
[0052] 1 H NMR (300MHz, CD 2 Cl 2 ,δ): 8.41 (s, 2H), 8.24 (s, 2H), 7.68 (s, 2H), 7.14-6.90 (m, 16H).
[0053] MS(EI)m / z:[M] + calcd for C 35 h 22 N 4 o 3 , 546...
Embodiment 3
[0055] The synthetic route of target compound 3 is as follows:
[0056]
[0057] Add raw material 3 (2.09g, 5mmol), phenoxazine (2.2g, 12mmol), palladium acetate (90mg, 0.4mmol) and tri-tert-butylphosphine tetrafluoroborate (0.34g, 1.2mmol) in 100mL two-necked flask mmol), then sodium tert-butoxide (1.16 g, 12 mmol) was added into the glove box, and 60 mL of toluene previously dehydrated and deoxygenated was poured into the glove box under an argon atmosphere, and reacted at 120° C. for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 3:2) to obtain 1.3 g of compound 3 as a green powder, yield 48%.
[0058] 1 H NMR (300MHz, CD 2 Cl 2 ,δ): 8.17 (s, 2H), 7.72 (d, J=6.3Hz, 2H), 7.14-6.90 (m, 16H), 6.75 (d, J=6.6Hz, 2H).
[0059] MS(EI)m / z:[M] + calcd for C 35 h 22...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap