Citrullinated antigen modified peptide and its application
A citrullinated, antigen-based technology, applied in the field of citrullinated antigen-modified peptides, can solve the problems of increased infection risk, expensive biological agents and small-molecule targeted drugs, and difficulty in controlling lesions in a targeted manner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
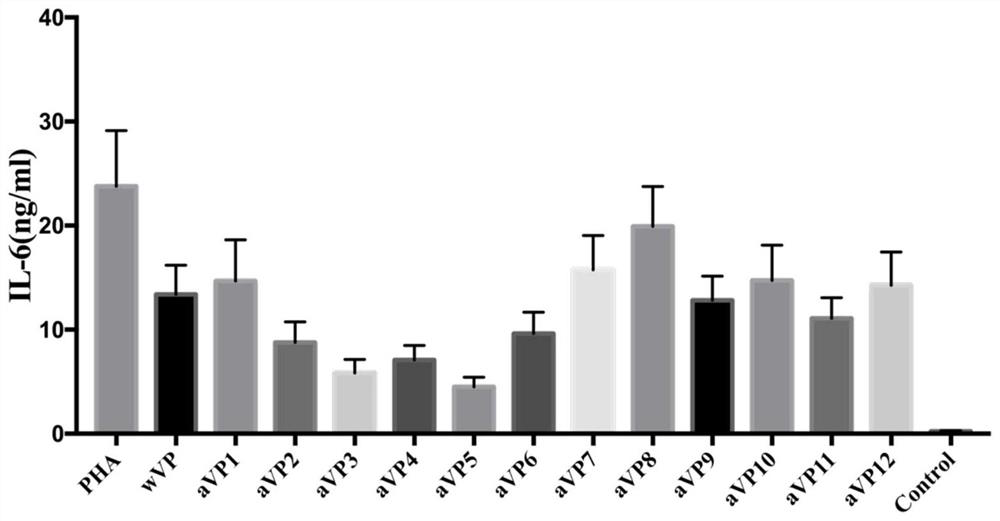
[0042] For the screening of citrullinated antigen-modified peptides, a total of 12 citrullinated antigen-modified peptides were designed, which are:
[0043] aVP1 (SEQ ID No. 6: SAVEL-Cit-SSVPGVR);
[0044] aVP2 (SEQ ID No. 7: SAVDL-Cit-SSVPGVR);
[0045] aVP3 (SEQ ID No. 3);
[0046] aVP4 (SEQ ID No. 8: SAVGL-Cit-SSVPGVR);
[0047] aVP5 (SEQ ID No. 4);
[0048] aVP6 (SEQ ID No. 9: SAVRL-Cit-FSVPGVR);
[0049] aVP7 (SEQ ID No. 10: SAVRL-Cit-SSVEGVR);
[0050] aVP8 (SEQ ID No. 11: SAVRL-Cit-SSVKGVR);
[0051] aVP9 (SEQ ID No. 12: SAVRL-Cit-SSVWGVR);
[0052] aVP10 (SEQ ID No. 13: SAVEL-Cit-WSVPGVR);
[0053] aVP11 (SEQ ID No. 14: SAVDL-Cit-SSVRGVR);
[0054] aVP12 (SEQ ID No. 15: SAVAL-Cit-KSVFGVR);
[0055]Prototype peptide (SEQ ID No. 16: SAVRL-Cit-SSVPGVR).
[0056] according to figure 1 It can be seen that under the stimulation of the prototype peptide, the level of IL-6 produced by peripheral blood PBMCs of patients with rheumatoid arthritis increases. However,...
Embodiment 2
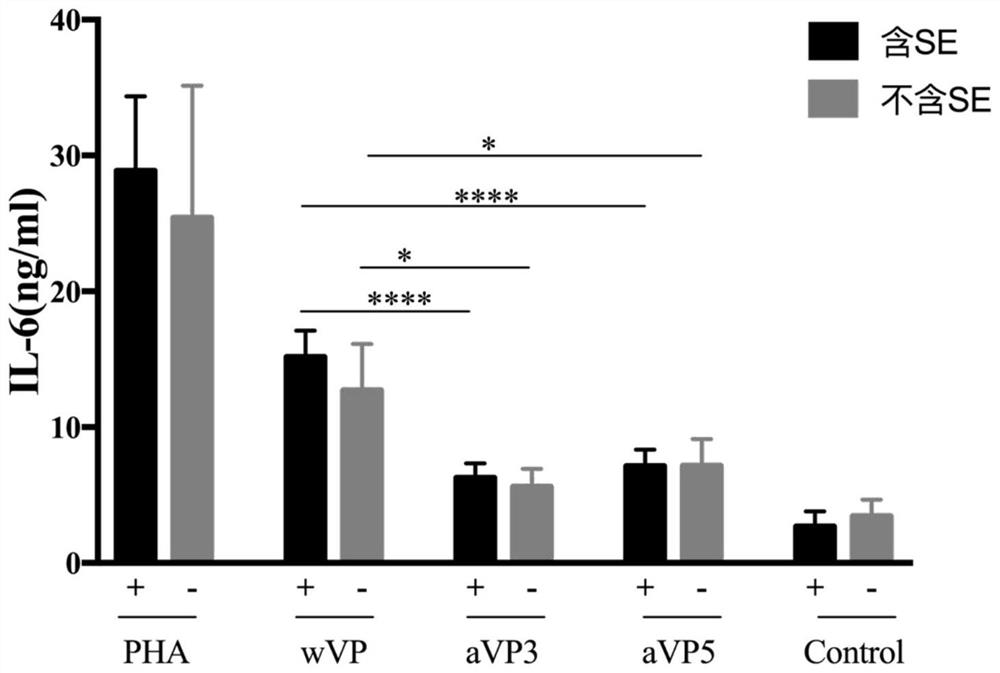
[0062] Hyporesponsiveness of citrullinated antigen-modified peptides in peripheral blood T cells of patients with rheumatoid arthritis.
[0063] In an embodiment of the present invention, the reactivity of peripheral blood T cells of patients with rheumatoid arthritis to two citrullinated antigen-modified peptides, aVP3 and aVP5, was studied, and the expression of the inflammatory cytokine IL-6 stimulated by the allosteric peptide was detected. produce level changes.
[0064] Starting with 4 mL of each patient's blood, blood was added to 4 mL of Ficoll-Paque PLUS in a 15 mL centrifuge tube that was centrifuged at 1200 rpm for 30 minutes. Extract the corresponding mononuclear cell (PBMC) layer. Subsequently, the cells were washed twice with 15 mL of 1×PBS, and they were centrifuged at 1200 rpm after each wash. Finally, the cell pellet was resuspended in RPMI1640 containing 10% fetal bovine serum plus penicillin (100 U / mL), streptomycin (100 μg / mL), 25 mM / L HEPES and 2 mM L-gl...
Embodiment 3
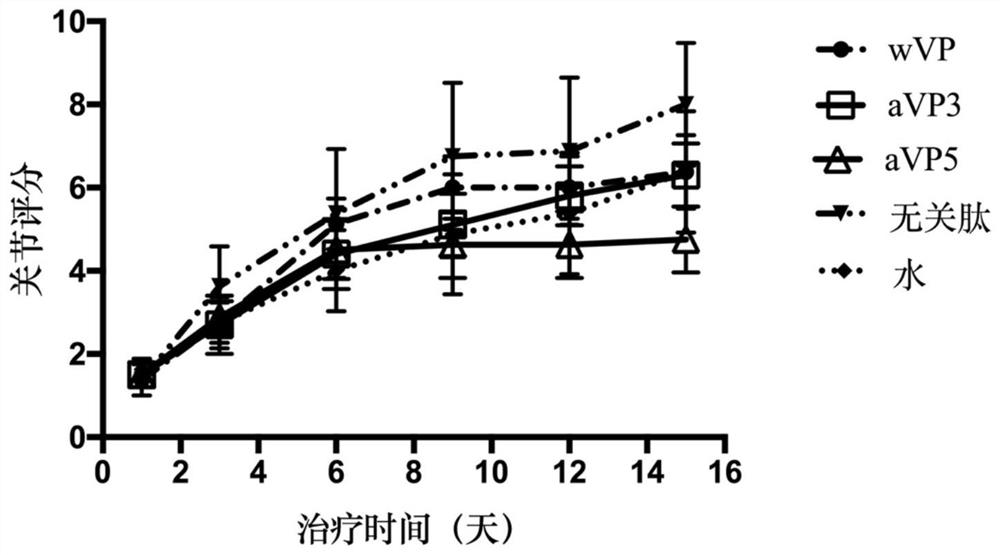
[0070] Therapeutic effects of aVP3 and aVP5 allosteric peptides were evaluated in an animal model of bovine type II collagen (CII)-induced arthritis.
[0071] In this example, an irrelevant peptide (iP) was added in addition to wVP, aVP3 and aVP5. The peptide segment of the irrelevant peptide is the reverse sequence Myr-RVGPVSS-Cit-LRVAS (SEQ ID No. 5) of wVP.
[0072] The experimental arthritis model in the present invention adopts the internationally recognized collagen-induced arthritis (CIA) model. The experimental animals were inbred DBA / 1 mice. The mice used in this experiment were from Beijing Huafukang Biotechnology Co., Ltd. All mice were raised in the Animal Experiment Center (SPF level) of Peking University People's Hospital. All experimental mice were male mice aged 6-8 weeks. All procedures involving animals in the experiment were carried out in accordance with the regulations on the management of experimental animals in my country.
[0073] Purchase bovine t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com