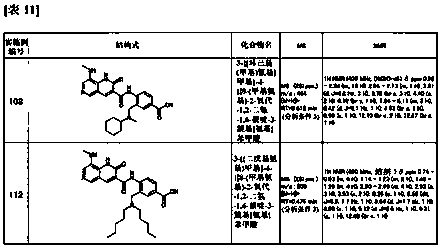
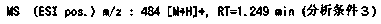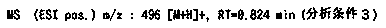2(1H)-quinolinone derivative
A compound, morpholino-based technology, applied in the field of 2(1H)-quinolinone derivatives or its salts, can solve the problem of insufficient effect of Gram-negative bacteria, high eukaryotic toxicity, unknown GyrB/ParE inhibitory effect, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 18
[0379] Production Example 1 8-(Methylamino)-2-oxo-1,2-dihydroquinoline-3-carboxylic acid
[0380] 1-(1) Ethyl 8-(methylamino)-2-oxo-1,2-dihydroquinoline-3-carboxylate.
[0381] [chemical formula 18]
[0382]
[0383] Diethyl malonate (434 μL) and piperidine (263 μL) were added to a solution of 2-amino-3-(methylamino)benzaldehyde (308 mg) in ethanol (4 mL), and heated and stirred at 75° C. for 8 hours. After allowing to cool to room temperature, the precipitated solid was collected by filtration to obtain the title compound (291 mg) as a yellow solid.
[0384]
[0385] 1-(2) 8-(Methylamino)-2-oxo-1,2-dihydroquinoline-3-carboxylic acid
[0386] [chemical formula 19]
[0387]
[0388] In tetrahydrofuran (12 mL) solution of 8-(methylamino)-2-oxo-1,2-dihydroquinoline-3-carboxylic acid ethyl ester (291 mg) obtained in Production Example 1-(1), add 1M aqueous sodium hydroxide solution (12 mL), stirred at room temperature for 8.5 hours. The reaction solution was neutralize...
manufacture example 2
[0390] Production Example 2 6-fluoro-8-(methylamino)-2-oxo-1,2-dihydroquinoline-3-carboxylic acid
[0391] 2-(1) [5-fluoro-3-(methylamino)-2-nitrophenyl]methanol
[0392] [chemical formula 20]
[0393]
[0394] A 33% methylamine-ethanol solution (27.3 mL) was added to a solution of (3,5-difluoro-2-nitrophenyl)methanol (20.72 g) in ethanol (50 mL) at room temperature, followed by stirring for 2 hours. The generated insoluble matter was collected by filtration, washed with ethanol and ether to obtain the title compound (12.96 g) as a yellow solid.
[0395]
[0396] 2-(2)5-Fluoro-3-(methylamino)-2-nitrobenzaldehyde
[0397] [chemical formula 21]
[0398]
[0399] In the chloroform (2840mL) solution of [5-fluoro-3-(methylamino)-2-nitrophenyl]methanol (23.66g) obtained by Production Example 2-(1), add carbon dioxide at 50°C Manganese (51.38g), heated and stirred for 7 hours. The insoluble matter was separated by filtration, and the filtrate was concentrated under redu...
manufacture example 38
[0416] Production Example 3 8-(Methylamino)-2-oxo-1,2-dihydro-1,7-naphthyridine-3-carboxylic acid
[0417] tert-butyl 3-(1) [2-[benzyl(methyl)amino]pyridin-3-yl]carbamate
[0418] [chemical formula 25]
[0419]
[0420] In a solution of N2-benzyl-N2-methylpyridine-2,3-diamine (20.4 g) in tetrahydrofuran (400 mL), add a solution of 1.9 M (trimethylsilyl)amide sodium in tetrahydrofuran (THF) under ice-cooling ( 111mL) and di-tert-butyl dicarbonate (23.0g), stirred under nitrogen atmosphere at room temperature for 2 hours. The reaction solution was diluted with water and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by column chromatography (NH silica gel cartridge, hexane:ethyl acetate=88:12) to obtain the title compound (26.8 g) as a red oily substance.
[0421]
[0422] tert-butyl 3-(2)[2-[benzyl(methyl)amino]-4-formylpyridin-3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com