Compositions for treatment of drug-resistant tumors and methods of use thereof
A therapeutic and compositional technology, applied in the field of composition and its application in the treatment of drug-resistant tumors, can solve the problem that the root cause is not very clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] method
[0156] siRNA and crRNA reagents
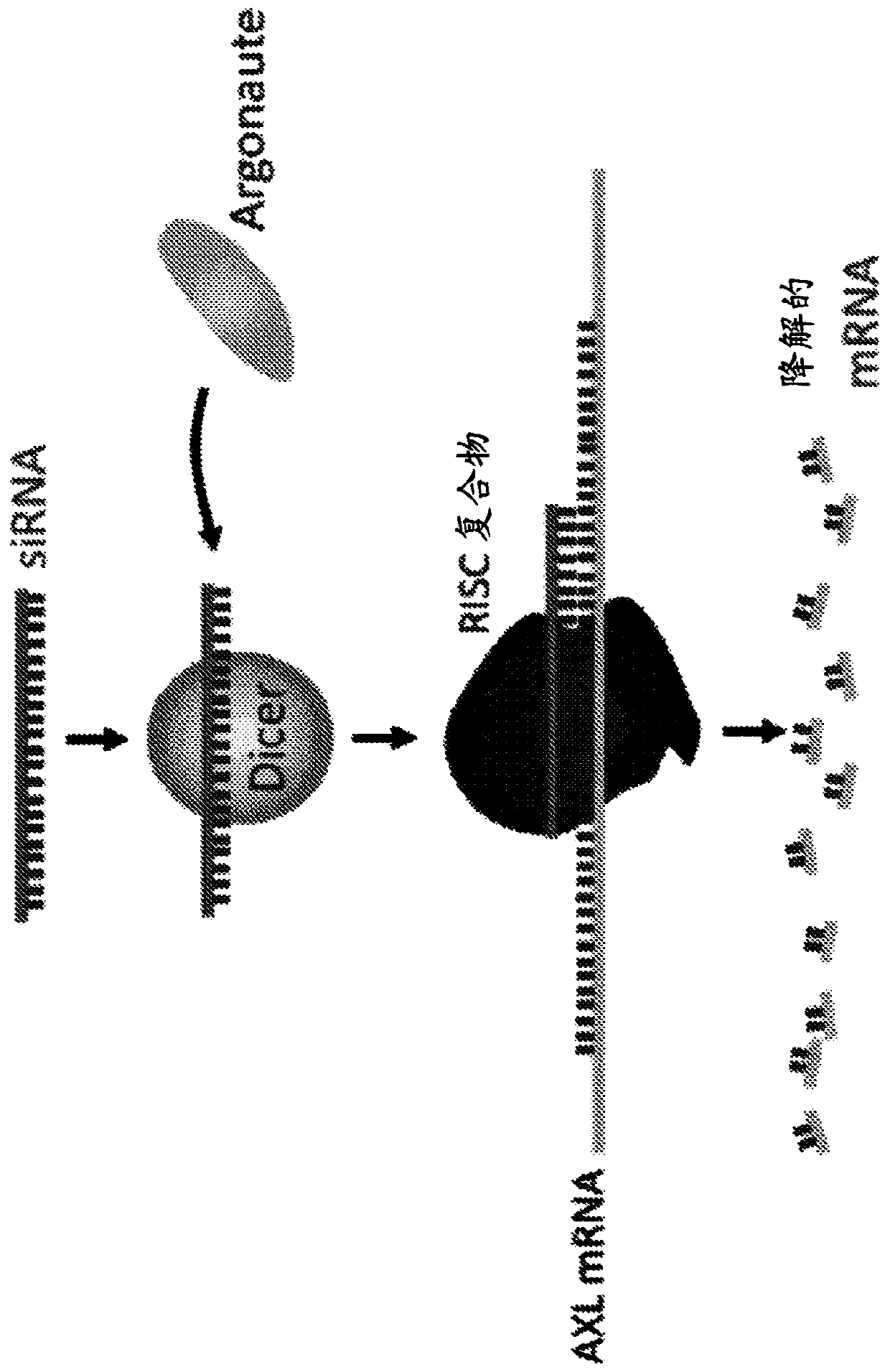
[0157] To inhibit protein expression of certain pathways, siRNAs were used to target and downregulate AXL, FN14, and KRAS mRNAs. For this purpose, AXL siRNA (sense strand: 5'GGAACUGCAUGCUGAAUGAUU 3') (SEQ ID NO:2), FN14 siRNA (sense strand: 5'CUCAGAUGUCCUGAAAUUCCAUU 3') (SEQ ID NO:3) and G12S mutated KRAS siRNA were used (Sense strand: 5'CAGCUAAUUCAGAAUCAUUUU 3') (SEQ ID NO:4).
[0158] A disulfide group was introduced at the 5' position of the oligonucleotide sequence for conjugation purposes. This dithio group is deprotected or reduced to -SH for further conjugation. An example of a disulfide structure such as "S-S-oligonucleotide" is:
[0159]
[0160] For prediction of conjugated siRNA and in vitro fluorescence imaging, Cy5 was conjugated to the 3' end of the antisense strand of AXL siRNA.
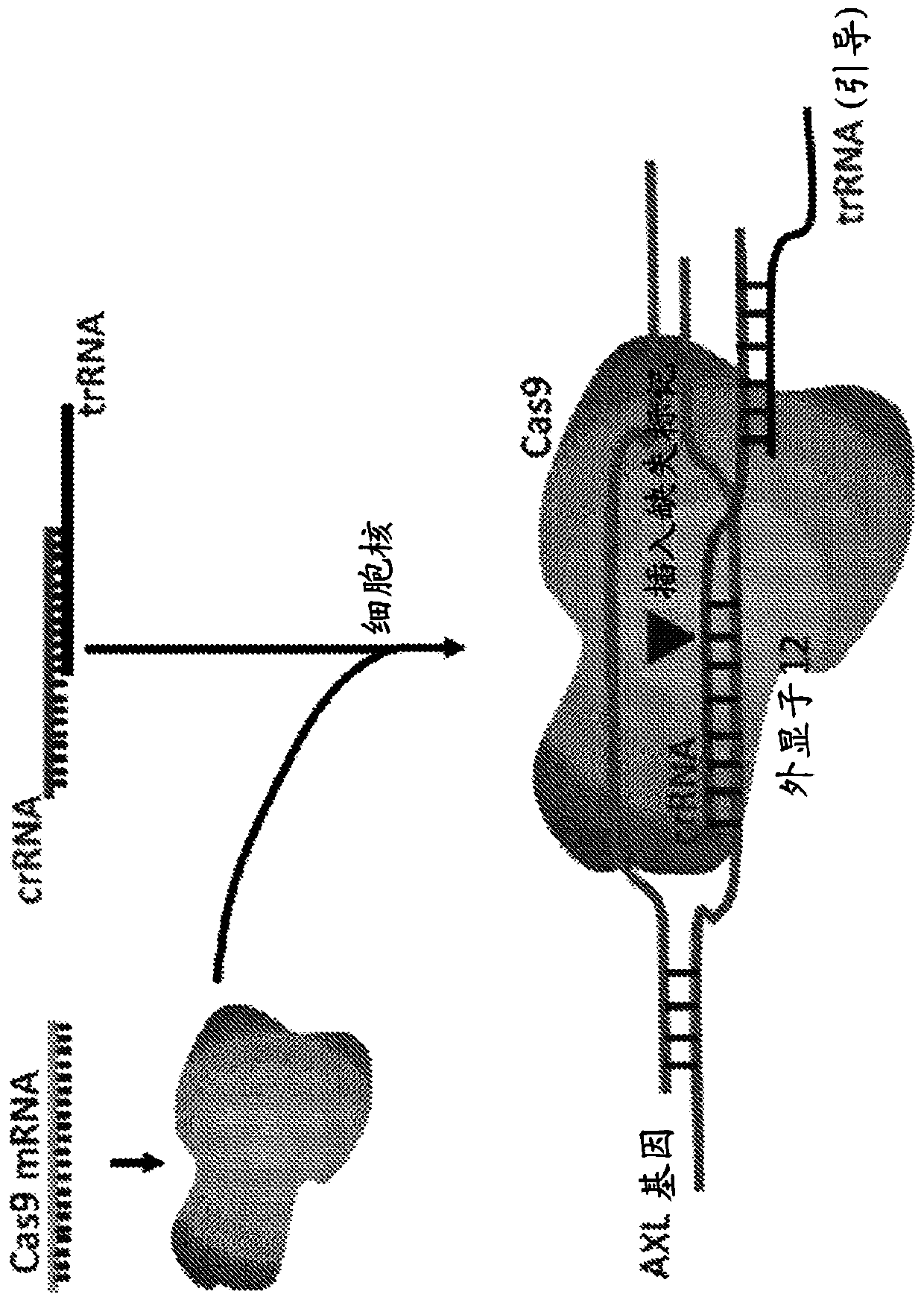
[0161] AXL crRNA was designed to target TTCAGTGGTCCGACGACTGT (SEQ ID NO:5) and PAM:hg38|-chr19:41243678-41243700AGG at genomic...
Embodiment 2
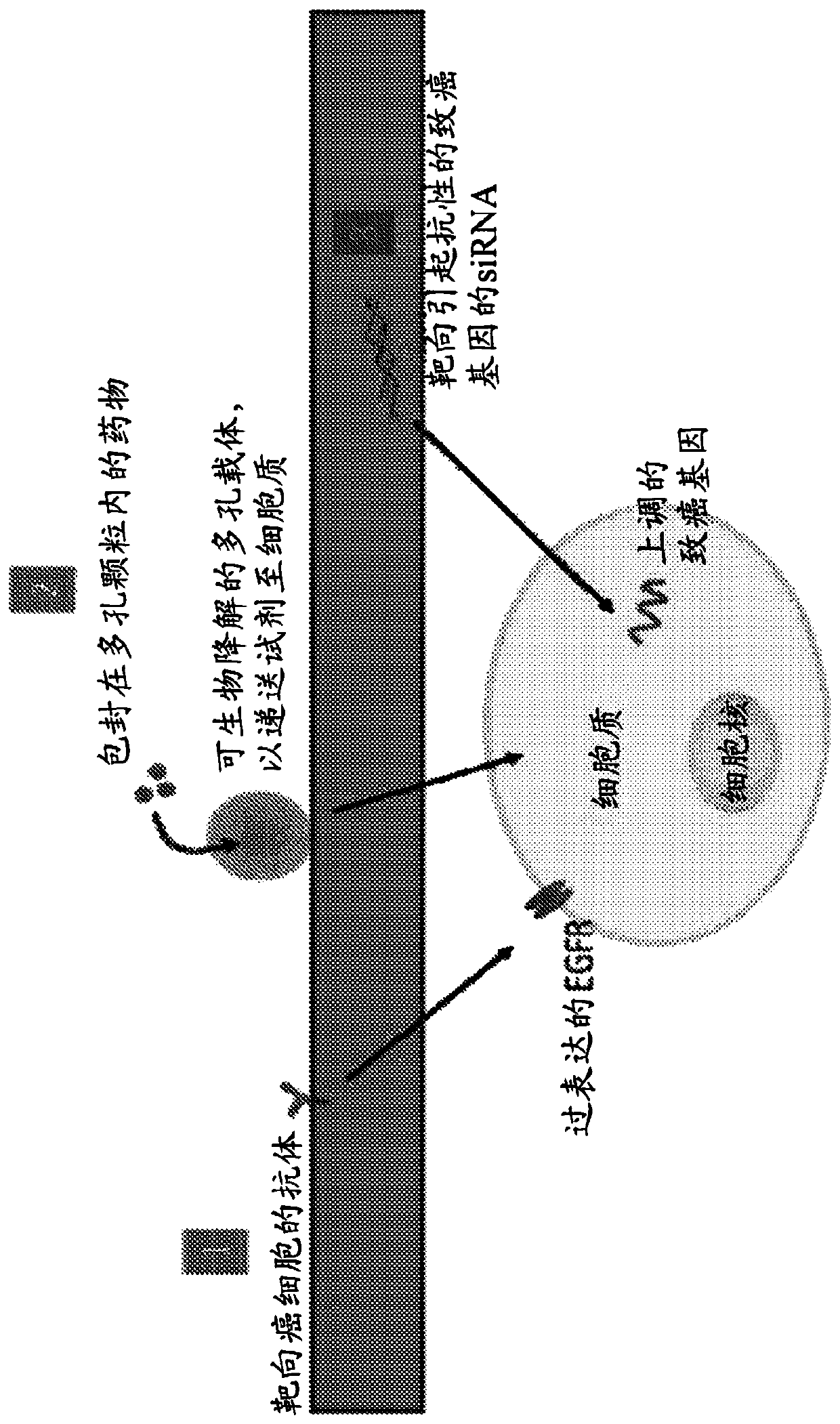
[0200] In vitro targeting of AXL-FN14 using nanoparticles
[0201] Based on the results of siRNA+drug toxicity studies, the down-regulation of the AXL-FN14 axis in H820 cells by treatment with siRNA-conjugated nanoconstructs was investigated. For this study, the toxicity of gelatin nanoparticles in H820 and HCC827 cells was initially tested. The results indicated that the particles after glutaraldehyde quenching were non-toxic (Figure 45). The study also demonstrated the ability of gelatin nanoparticles to deliver drugs by encapsulation in HCC827 drug-sensitive cells (Figure 45).
[0202] Cytotoxicity assays also confirmed that the synthetic siRNA conjugates were non-toxic (Figure 46). In the next step, drug resensitization using the dual siRNA AXL-FN14 construct was investigated in H820 cells. Similar results to siRNA-based treatments were obtained (Figure 47). The results demonstrate that nanoparticles can be used to target these pathways and may be an important step in ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap