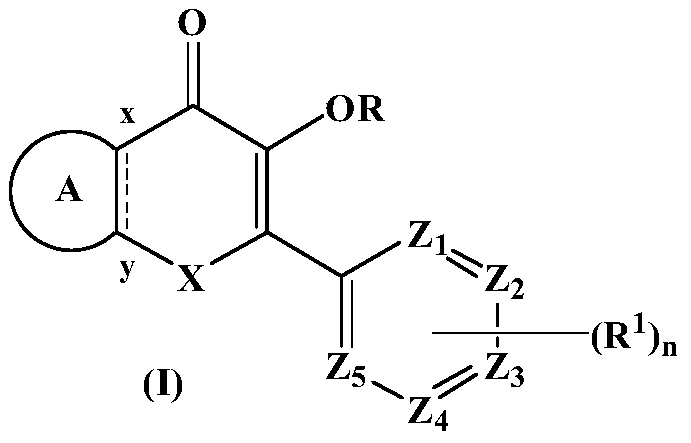
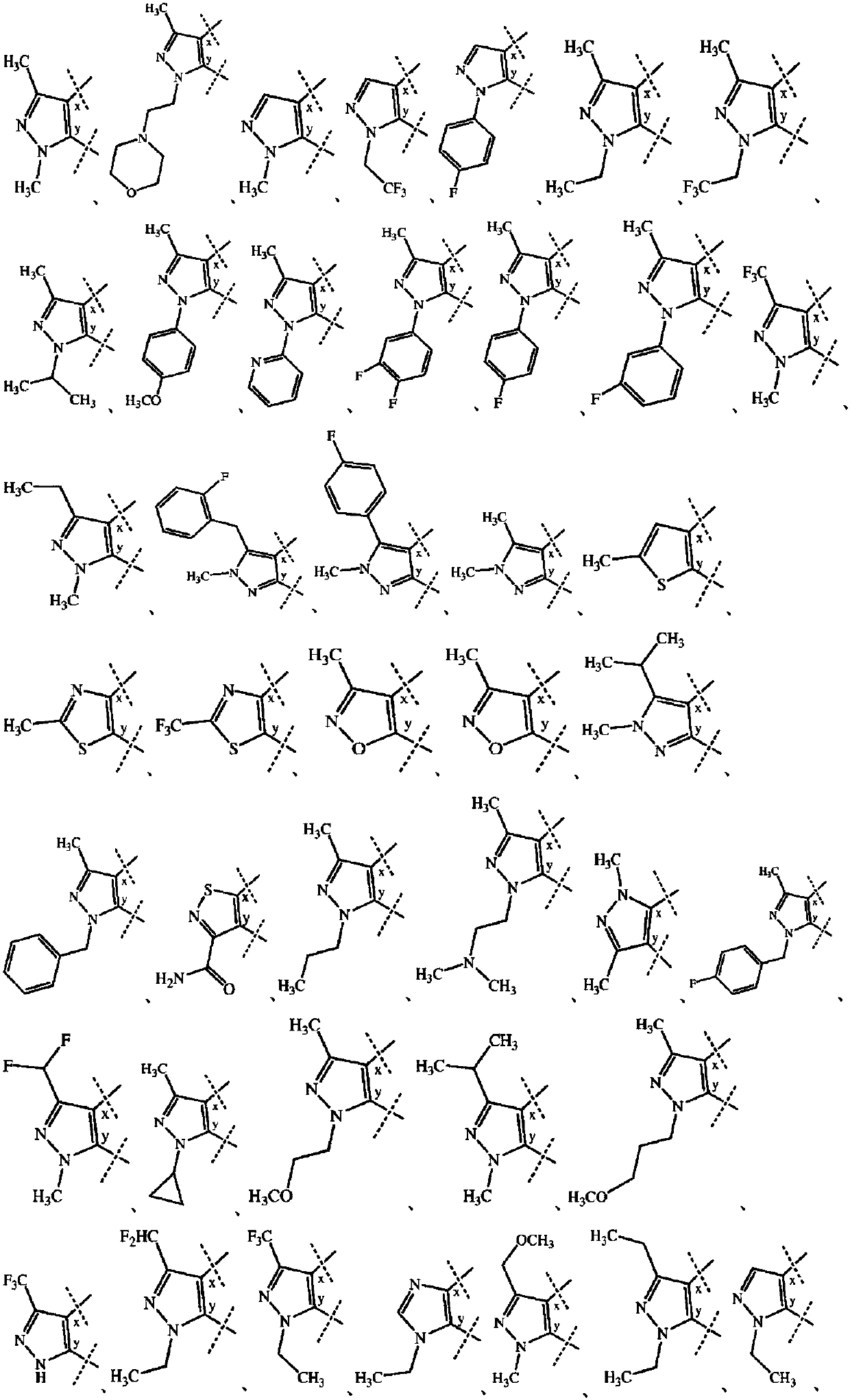
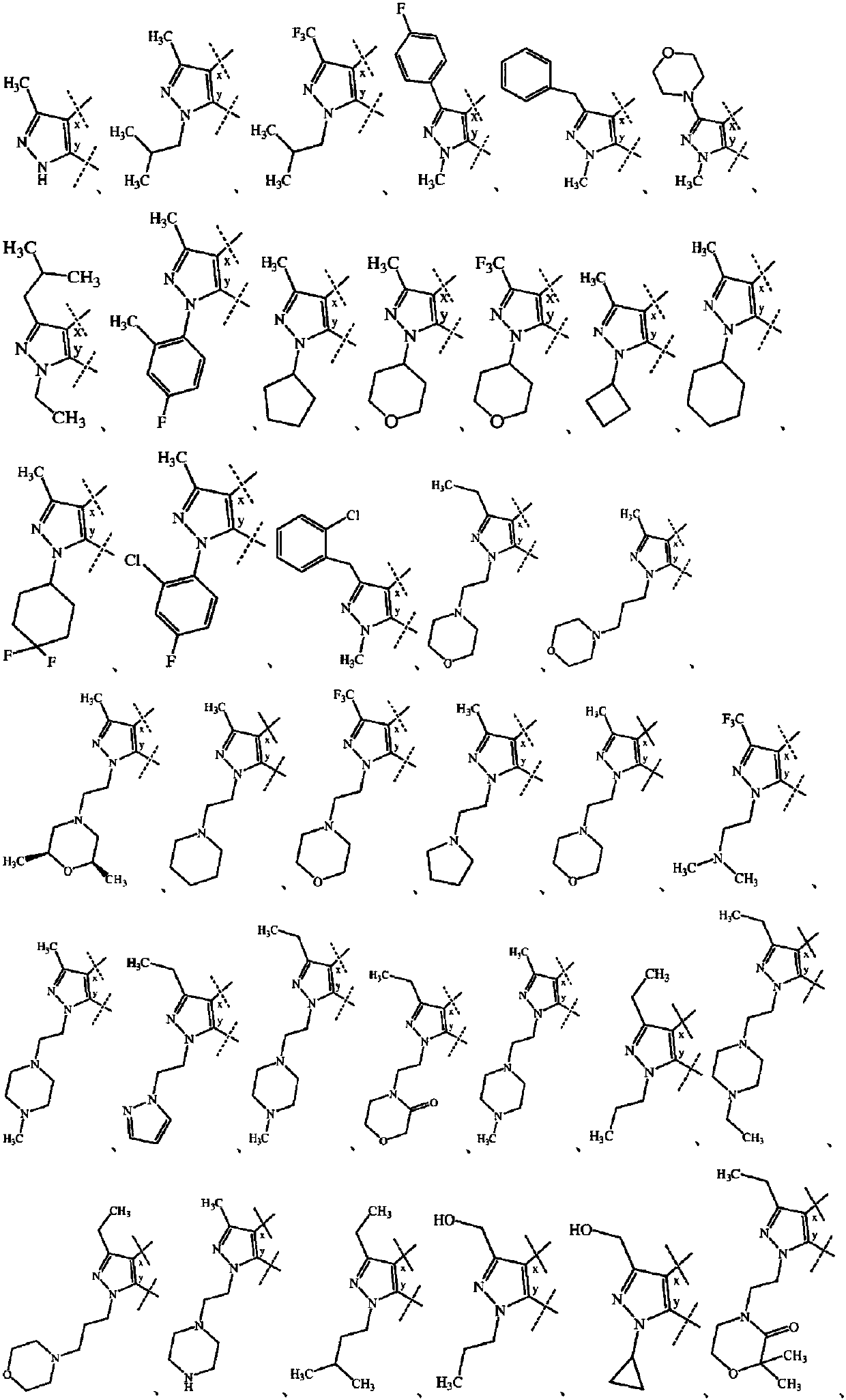
Substituted bicyclic heterocyclic compounds as nadph oxidase inhibitors
A compound, heterocyclic group technology, applied in the field of substituted bicyclic heterocyclic compounds as NADPH oxidase inhibitors, can solve problems such as destroying and changing the function of target molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1770] Synthesis of 6-(2-chlorophenyl)-5-hydroxy-1,3-dimethyl-1,7-dihydro-4H-pyrazolo[3,4-b]pyridin-4-one
[1771]
[1772] 5-Amino-1,3-dimethyl-1H-pyrazole-4-carboxylic acid 2-(2-chlorophenyl)-2-oxoethyl ester (590mg, 1.91mmol) and polyphosphoric acid (6.0mL ) mixture was heated to 120°C for 3h. The reaction mixture was cooled to RT and neutralized with 1N sodium hydroxide. The reaction mixture was extracted with ethyl acetate (3 x 100 mL), and the organic layer was washed with water (100 mL). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel to afford 112 mg of the title product as a solid. 1 H NMR (300MHz, DMSO-d 6 ): δ2.52(s, 3H), 3.80(s, 3H), 7.45-7.60(m, 4H), 7.89(s, 1H), 11.62(br s, 1H); APCI(m / z) 290( M+H) + .
[1773] Method B:
Embodiment 2
[1775] 6-(2-Chlorophenyl)-5-hydroxy-3-methyl-1-(2-morpholinoethyl)-1H-pyrazolo[3,4-b]pyridine-4(7H)- Ketone synthesis
[1776]
[1777] 5-amino-3-methyl-1-(2-morpholinoethyl)-1H-pyrazole-4-carboxylic acid 2-(2-chlorophenyl)-2-oxoethyl ester (intermediate -91, 4.0 g, 9.85 mmol) in concentrated sulfuric acid (30 mL) was stirred at 80 °C for 2 h. The reaction mixture was cooled to RT and quenched with ice-cold water (35 mL). The precipitated solid was filtered and dried well to give 1.56 g of the desired product. 1 HNMR (300MHz, DMSO-d 6 ): δ2.39-2.52(m, 7H), 2.69(t, J=7.0Hz, 2H), 3.16-3.41(m, 4H), 4.27(s, 2H), 7.49-7.63(m, 4H), 7.91 (br s, 1H), 11.89 (br s, 1H); ESI (m / z) 389 (M+H) + .
[1778] The preparations of Examples 3-190, 193-194, 198, 200, 202-213, 215 and 217-218 given in Table 1 were carried out according to any of the procedures mentioned above. The structural formula, chemical name, 1 H NMR and MS data.
[1779] Table 1 : structure, chemical name, 1 H...
Embodiment 191
[1822] 6-(2,6-difluorophenyl)-5-hydroxy-1-methyl-3-(morpholinomethyl)-1H-pyrazolo[3,4-b]pyridine-4(7H) -ketone
[1823]
[1824] To a solution of morpholine (28.3 mg, 0.325 mmol) in anhydrous THF (2 mL) was added (6-(2,6-difluorophenyl)-5-hydroxy-3-(hydroxymethyl)-1- Methyl-1H-pyrazolo[3,4-b]pyridin-4(7H)-one) (50 mg, 0.162 mmol) and triphenylphosphine (61.8 mg, 0.23 mmol). The reaction was then cooled at 0° C., and diisopropyl azodicarboxylate (DIAD) (46.8 μL, 0.236 mmol) was added dropwise thereto. The reaction mixture was stirred at RT for 18 h. The mixture was quenched with water (2 drops), then evaporated and purified by column to give 18 mg of the title product. 1 H NMR (400MHz, DMSO-d6 ): δ2.77-2.81(m, 4H), 3.72-3.76(m, 4H), 3.80(s, 3H), 4.00(s, 2H), 7.14-7.20(m, 2H), 7.47-7.53(m , 1H), 8.58 (brs, 1H); ESI (m / z) 377 (M+H) + .
[1825] The preparation of Examples 192, 195-197, 199, 201, 214, 216 and 219-224 given in Table 2 was carried out according to any of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com