Maytansine polypeptide conjugate as well as preparation method and application thereof
A technology of polypeptide coupling and maytansine, which is applied in the preparation methods of peptides, chemical instruments and methods, and peptides, etc., can solve the problems of insufficient ability to penetrate the membrane, poor water solubility, and poor selectivity of targeted peptides, so as to increase the anti-tumor effect. Efficacy, improvement of targeting, and reduction of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
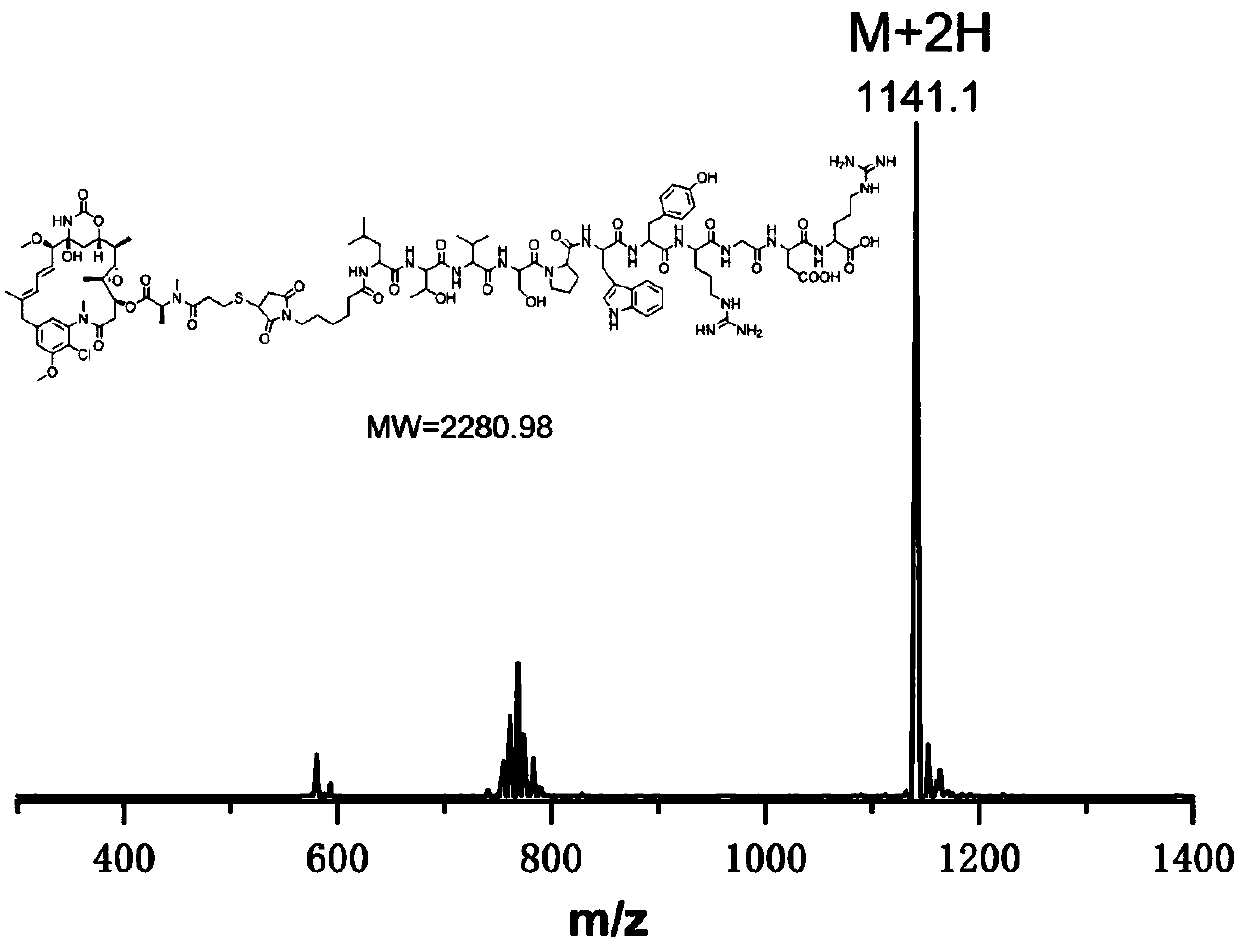
[0028] A maytansine polypeptide conjugate has the following structural formula:
[0029]
[0030] Using the solid-phase peptide synthesis method of the Fmoc strategy, the peptide synthesizer produced by CSBio Company was used to synthesize the targeted polypeptide of the present invention.
[0031] Selection of reagents used for synthesis:
[0032] (1) Carrier resin: Fmoc-Arg(Pbf)Wang, degree of substitution: 0.67
[0033] (2) Selected protected amino acids: Fmoc-Leu-OH, Fmoc-Thr(tBu)-OH, Fmoc-Val-OH, Fmoc-Ser(tBu)-OH, Fmoc-Pro-OH, Fmoc-Trp(Boc )-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Arg(pbf)-OH, Fmoc-Gly-OH, Fmoc-Asp(OtBu)-OH, N-maleimidocaproic acid, reacting A 3-fold excess of protected amino acids was used.
[0034] (3) The deprotection reagent used in the present invention is: piperidine / N,N-dimethylformamide, the ratio is 20:80.
[0035] (4) The coupling reagent used in the present invention is: DIEA / HBTU.
[0036] (5) The cleavage reagent used in the present invention is: ...
Embodiment 2
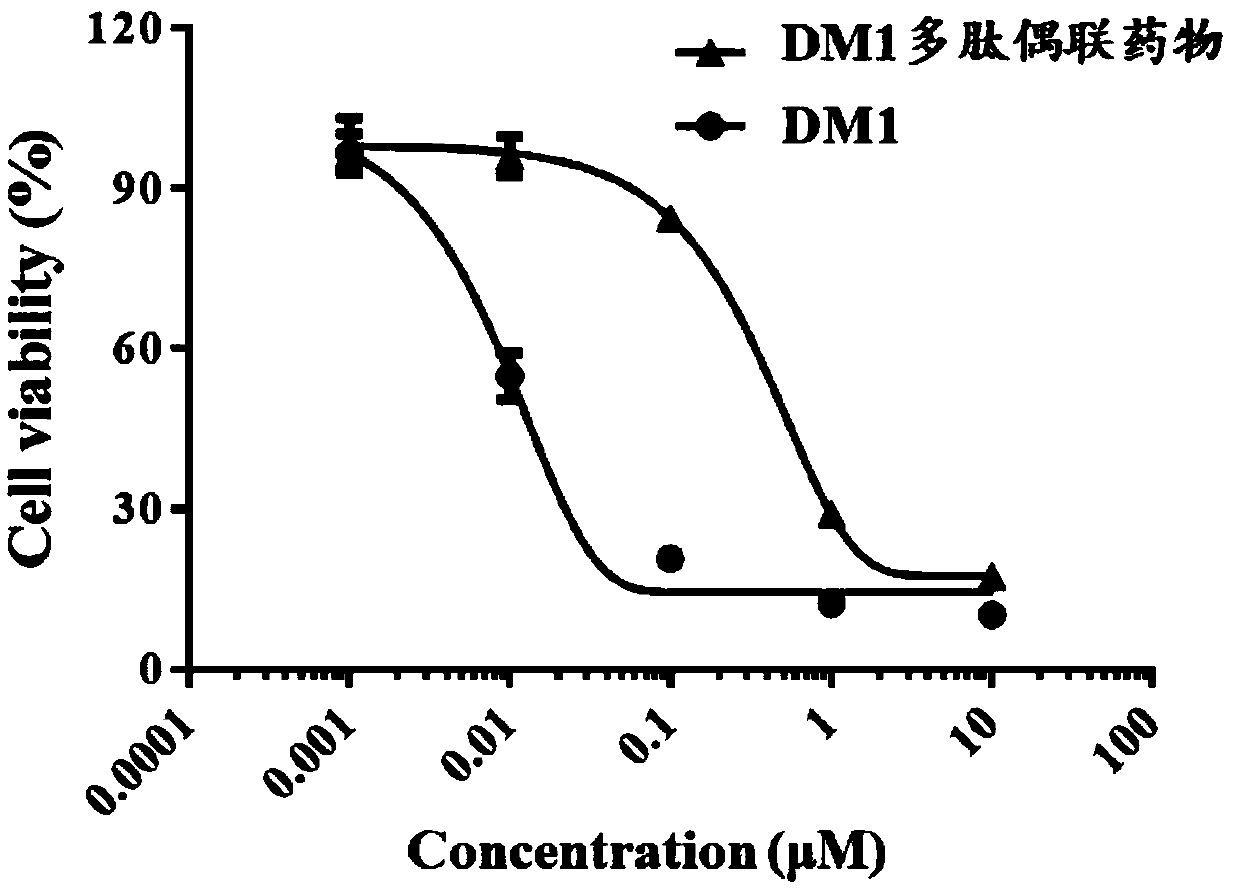
[0046] A maytansine polypeptide conjugate has the following structural formula:
[0047]
[0048] The preparation method of the above polypeptide drug conjugate precursor is as follows:
[0049] 1) Synthesis of MCC-LTVSPWYRGDR:
[0050] Using the solid-phase peptide synthesis method of the Fmoc strategy, the amino acid sequence was synthesized sequentially, and 4-(N-maleimidomethyl)cyclohexylcarboxylic acid was also directly connected to the peptide fragment through solid-phase synthesis to obtain MCC-LTVSPWYRGDR. The reaction was initiated with 0.2 g of Fmoc-Arg(Pbf) Wang resin, 120 mg of the crude polypeptide was obtained after cleavage, and 44 mg was obtained by purification in the preparative liquid phase, with a total yield of 21%.
[0051] 2) Synthesis of DM4-MCC-LTVSPWYRGDR:
[0052]Dissolve DM4 (50mg, 0.06mmol, 3eq), MCC-LTVSPWYRGDR (31mg, 0.02mmol) in 1mL THF, then add 1mL of PBS buffer to adjust the pH value of the system to 6-8, then stir the reaction at room t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ld50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap