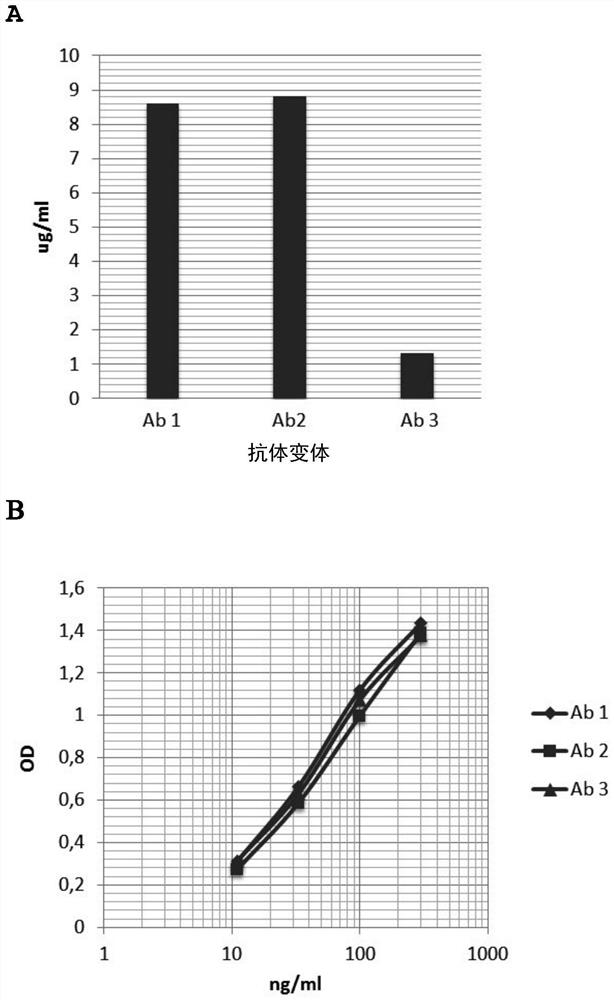
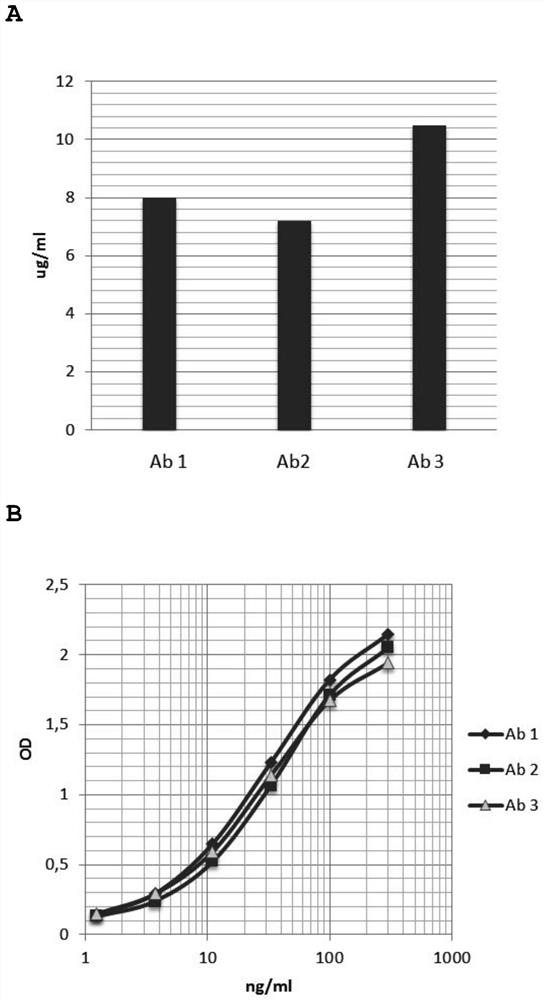
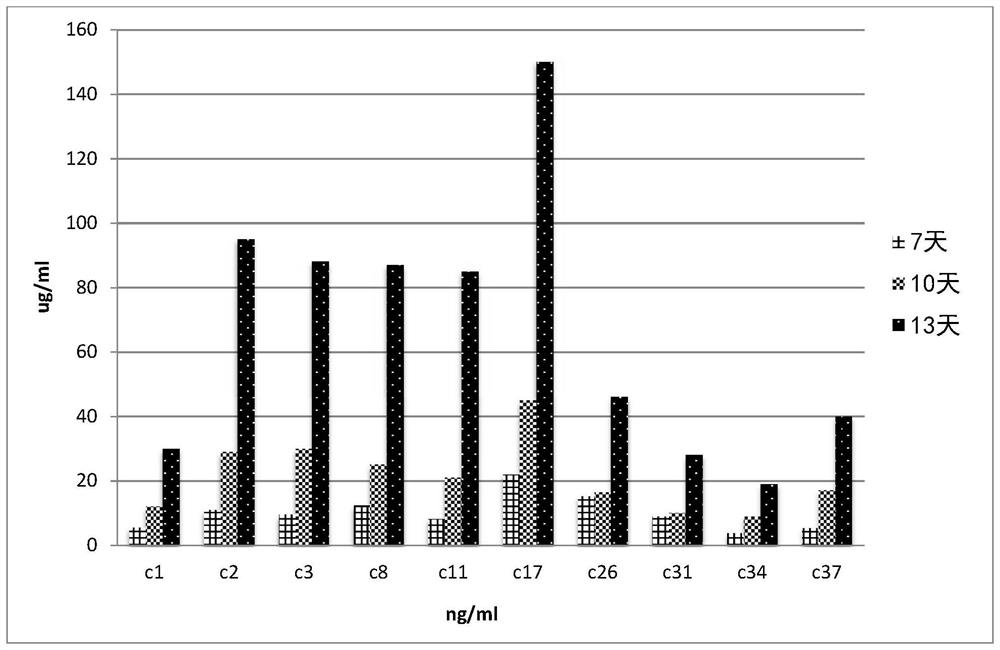
Humanized and de-immunized antibodies
A humanized antibody and antibody technology, applied in the field of humanized and deimmunized antibodies, can solve problems such as insufficient antibody, impossible to establish CMC production, and inability to establish stable cell clones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0332] Example
[0333] 1. To produce N3pE-Aβ specific antibody by humanization method
[0334] The humanized and deimmunized antibodies of the present invention are humanized and deimmunized forms of monoclonal mouse antibodies produced by the hybridoma cell line Aβ6-1-6 (Accession No. DSM ACC 2924), which hybridoma cells The line Aβ6-1-6 is described in WO2010 / 009987. Humanization was performed as described in WO 2017 / 009459. Working Example 1 disclosed on pages 58-60 of WO 2017 / 009459 is incorporated herein by reference.
[0335] 2. RNA Isolation and cDNA Synthesis
[0336] As a source of constant sequences, RNA from human B cells was isolated by lysing 500 [mu]l whole blood with 5 ml 1x FACS Lysis Buffer (Becton Dickinson) for 10 min at room temperature. The lysate was centrifuged at 300 g for 5 minutes; the pellet was washed twice with PBS and then dissolved in 350 μl Nucleo RNA II in RA1 buffer (Macherey-Nagel) and 3.5 μl of 0.5M TCEP (SIGMA) was added. Isolate RN...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap