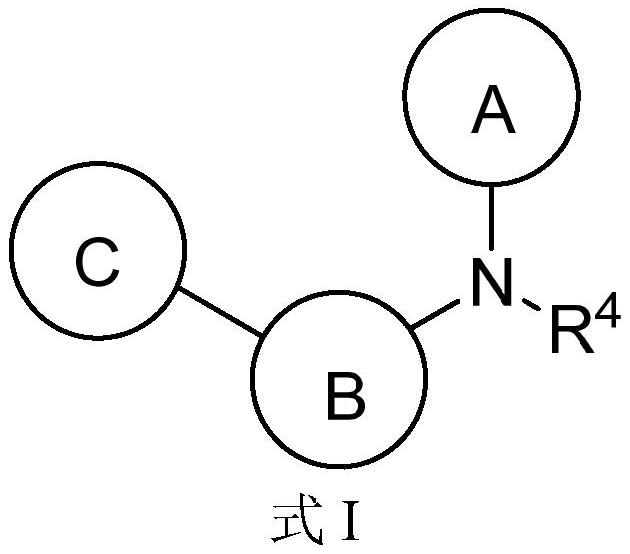
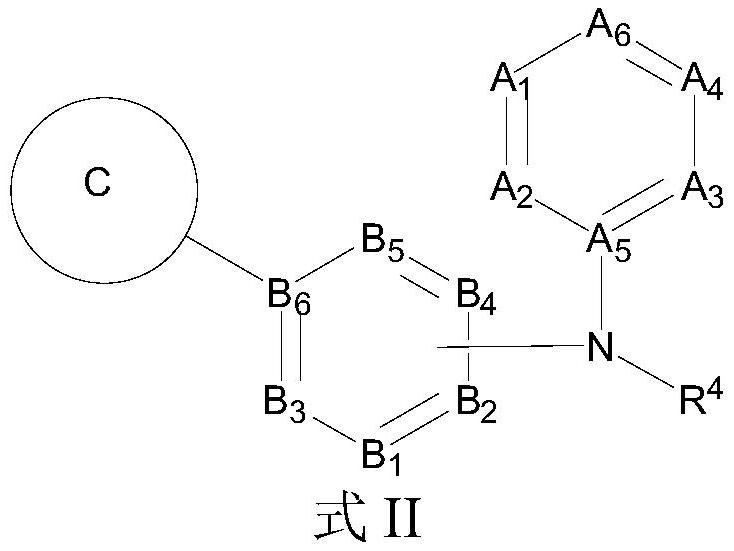
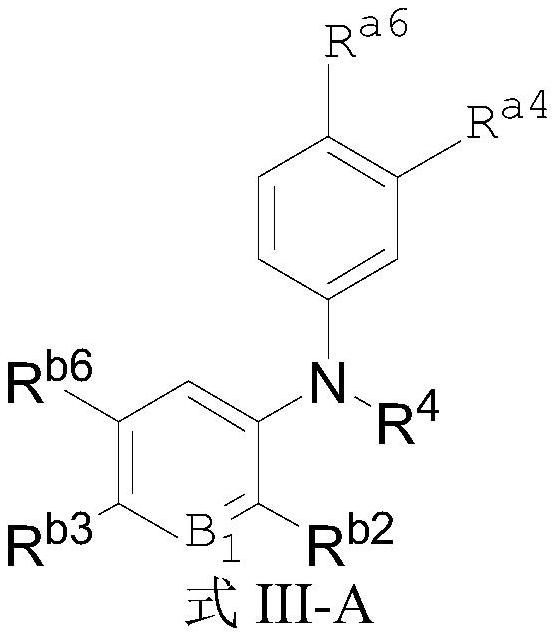
Aromatic amine compounds and their use in the preparation of AR and BRD4 dual inhibitors and regulators
A compound and inhibitor technology, applied in the directions of active ingredients of heterocyclic compounds, introduction of isotopes of heterocyclic compounds, introduction of isotopes into organic compounds, etc., can solve the problems of drug resistance and adverse reactions of small molecule drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 Synthesis N- (3-bromo-4- (1H-imidazole-1-yl) phenyl) -5- (3,5-dimethyl oxazole-4-yl) -N-ethyl- 2-metharmiline (Compound 31)
[0094]
[0095] first step:
[0096] 2-bromo-1-fluoro-4-iodobenzene (3.0 g, 10 mmol), imidazole (680 mg, 10 mmol) and cesium carbonate (4.9 g, 15 mmol) were sequentially added to 50 ml of DMA, and under nitrogen protection was heated to 120 ° C. After the reaction overnight, the TLC was monitored, naturally cooled to room temperature, extracted with 100 ml of ethyl acetate, saturated salt water (3 * 50 mL), dried over anhydrous sodium sulfate, concentrated, chromatographic column separation purification (PE / EA = 4 / 1 3.4 g of a compound 1- (2-bromo-4-iodobenzene) -1H-imidazole, yield: 95%.
[0097] Step 2:
[0098] (1) Synthesis of raw materials 5- (3,5-dimethyl alioxazole-4-yl) -2-methyl aniline (i.e., compound 31-2): (3,5-dimethyl oxazole) 4-yl) boric acid (16.9 g, 120 mmol), 5-bromo-2-methane (22.3 g, 120 mmol) is dissolved in 240 mL1...
Embodiment 2
[0102] Example 2 Synthesis 5- (3,5-dimethyl oxazole-4 -) - N-ethyl-2-methyl-N- (4- (5-methyl-1H-pyrazole-3- ) Phenyl) aniline (compound 97)
[0103]
[0104] first step:
[0105] 60% sodium hydride (80 mg, 2 mmol) was dissolved in 10 ml N, N-dimethylformamide, and 3- (4-bromo) -5-methyl-1H-pyrazole (236 mg, 1 mmol), room temperature The mixture was stirred for 10 min, 2- (trimethylsilyl) ethoxymethyl chloride (182 mg, 1.1 mmol) was added, and at room temperature for 3 hours, 10 mL washed, 10 ml of ethyl acetate extraction, and the organic layer was concentrated under reduced pressure, thin layer chromatography Purification of 341 mg of Compound 3- (4-bromo) -5-methyl-1 - (2- (trimethylsilicilicidal) ethoxy) methyl) -1H-pyrazole, yield: 93 %.
[0106] Step 2:
[0107] 5- (3,5-dimethyl oxazole-4 -) - 2-methamiline (202 mmol), dissolved in 5 ml 1,4-dioxane, add 3- (4-bromophenyl) Base) -5-methyl-1 - (2- (trimethylsilica) ethoxy) methyl) -1H-pyrazole (341 mg, 0.93 mmol), cesium carb...
Embodiment 3
[0112] Example 3 Synthesis 4- (4 - (5- (3,5-dimethyl otholactazole-4 - 2-methylphenyl) (ethyl) amino) phenyl) thiazole-2-amine (Compound 98)
[0113]
[0114] first step:
[0115] 4- (4-bromo) thiazole-2-amine (254 mg, 1 mmol) was dissolved in 10 ml of dichloromethane, and phthalic anhydride (140 mg, 0.96 mmol) was added, triethylamine (202 mg, 2 mmO) reflux is added. It was stirred for 6 hours, 10 mL 1N hydrochloric acid washed, 10 ml of ethyl acetate extraction, 10 ml of saturated sodium hydrogencarbonate, 10 ml of saturated salt water washed. The organic layer was concentrated under reduced pressure, and the crystal chromatography was separated from 360 mg of Compound 2- (4- (4-bromo-phenyl) thiazole-2-) isoindenam-1,3-diketone, yield: 94% .
[0116] Step 2:
[0117] 5- (3,5-dimethyl oxazole-4 -) - 2-methyl kethane (202 mmol), dissolved in 5 ml 1, 4-dioxane, added 2- (4- (4 - Bromobenzene) thiazole-2-) isoindenam-1,3-dilute (360 mg, 0.94 mmol), cesium carbonate (650 mg, 2 mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com