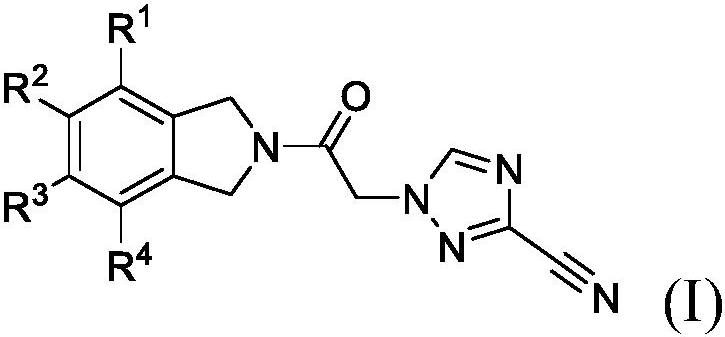
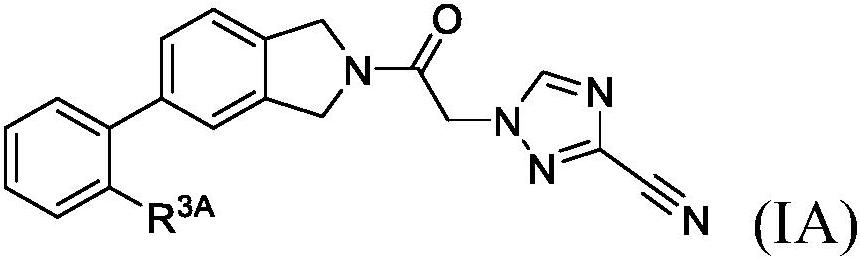
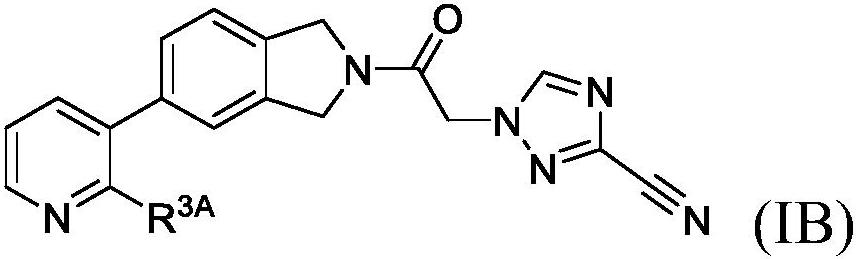
Cyanotriazole compounds and uses thereof
A compound, phenyl technology, applied in the field of cyanotriazole compounds, can solve problems such as reluctance to take miltefocin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0198] In the preparation of compounds of the invention, it may be desirable to protect remote functionality of intermediates. The need for such protection will vary depending on the nature of the remote functionality and the conditions of the preparation method. The need for such protection is readily ascertainable by those skilled in the art. For a general description of protecting groups and their use, see Greene, T.W. et al., Protecting Groups in Organic Synthesis, 4th ed., Wiley (2007). Protecting groups introduced in the preparation of compounds of the present invention, such as the trityl protecting group, may appear as one regioisomer, but may also exist as a mixture of regioisomers.
[0199] General Synthetic Route 1
[0200]
[0201] Reaction conditions:
[0202] a. Coupling the amide with a coupler in a polar aprotic solvent;
[0203] b. In inorganic bases such as Na 2 CO 3 or K 3 PO 4 Suzuki couplings were performed with various Pd catalysts in the prese...
example 1
[0367] Example 1: 1-(2-(5-(2-(difluoromethyl)pyridin-3-yl)isoindoline-2-yl)-2-oxoethyl)-1H-1,2, 4-triazole-3-carbonitrile
[0368]
[0369] Preparation of 3-bromo-2-(difluoromethyl)pyridine
[0370]
[0371] To a solution of 3-bromopicolinaldehyde (0.50 g, 2.68 mmol) in dichloromethane (10 mL) was added diethylamidosulfur trifluoride (DAST, 0.86 g, 5.37 mmol) dropwise at 0°C. The reaction mixture was stirred at 0 °C for 3 hours. It was then very carefully quenched with saturated sodium bicarbonate solution at 0 °C and extracted with dichloromethane (10 mL X 3). The combined organic layers were dried over sodium sulfate and concentrated under vacuum. The crude compound was purified by column chromatography using 0-30% EtOAc in n-Hexane to give the title compound (0.25 g, 44.84%) as a colorless liquid, Rf = 0.9 (30% EtOAc in n-Hexane); Rf = 0.90 (30% EtOAc in n-Hexane); 1 H NMR (300MHz, chloroform-d) δ8.65 (dd, J = 4.7, 1.4Hz, 1H), 7.98 (dd, J = 8.1, 1.2Hz, 1H), 7.32 ...
example 2
[0380] Example 2: 1-(2-(5-(3-Chloro-5-(trifluoromethyl)pyridin-4-yl)isoindoline-2-yl)-2-oxoethyl)-1H- 1,2,4-triazole-3-carbonitrile
[0381]
[0382] Preparation of 3-chloro-5-(trifluoromethyl)pyridin-2-amine
[0383]
[0384] Stir 2,3-dichloro-5-(trifluoromethyl)pyridine (1.0 g, 4.65 mmol) in aqueous NH in a sealed tube at 100 °C 3 (0.5 mL) for 12 h. The reaction mixture was concentrated to dryness and purified by combi flash column chromatography using 70% EtOAc in n-Hexane to give the title compound 0.8 g (90.0%), Rf = 0.1 (70% EtOAc in n-Hexane); 1 HNMR (300MHz, chloroform-d) δ8.23(d, J=1.8Hz, 1H), 7.70(d, J=1.8Hz, 1H); LC-MS m / z 196.9[M+H] + , retention time = 0.98 min (method D); HPLC: 95.32%, retention time = 6.53 min (method E).
[0385] Preparation of N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)pivalamide
[0386]
[0387] To a stirred solution of the precursor (400 mg, 2.04 mmol) in DCM (10 mL) was added pyridine (480 mg, 6.12 mmol) at 0 °C, followed by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap