Anti-trop2 nanobody and its preparation method and application
A technology of nanobodies and expression vectors, applied in the biological field to achieve the effect of novel sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
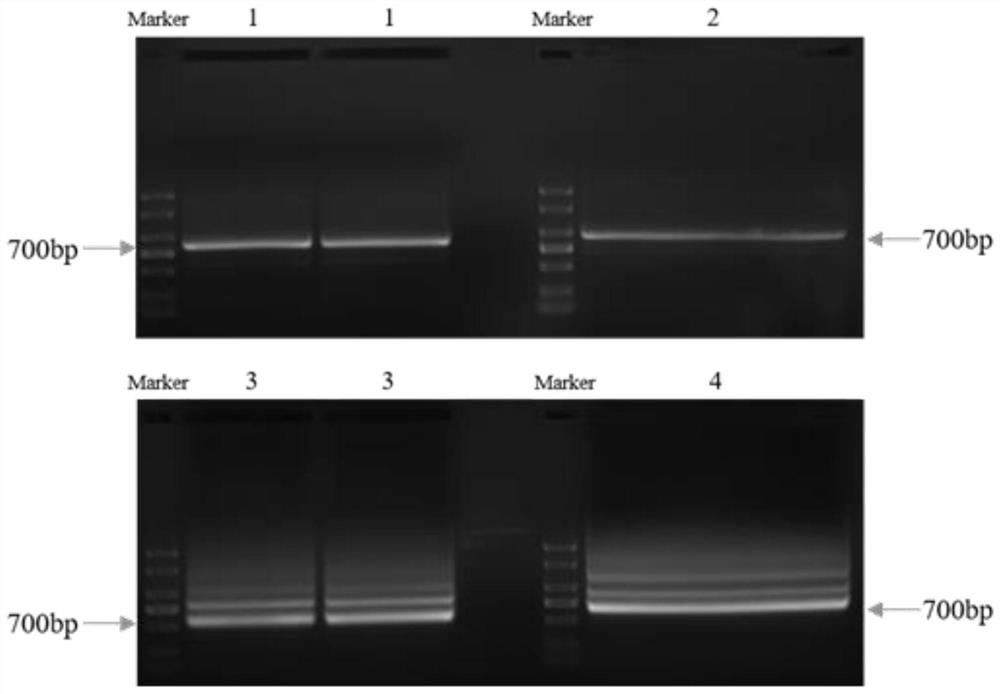
[0038] Example 1 Construction of camelid phage VHH display library
[0039] 1.1 Camel Immune
[0040] 取1mg人TROP2-Fc(公司自产,氨基酸序列为HTAAQDNCTCPTNKMTVCSPDGPGGRCQCRALGSGMAVDCSTLTSKCLLLKARMSAPKNARTLVRPSEHALVDNDGLYDPDCDPEGRFKARQCNQTSVCWCVNSVGVRRTDKGDLSLRCDELVRTHHILIDLRHRPTAGAFNHSDLDAELRRLFRERYRLHPKFVAAVHYEQPTIQIELRQNTSQKAAGDVDIGDAAYYFERDIKGESLFQGRGGLDLRVRGEPLQVERTLIYYLDEIPPKFSMKRLTEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK)蛋白与弗氏完全佐剂1:1等体积混匀并充分乳化,采用皮下多点注射的方式对一只健康成年骆驼进行第一 The first immunization; followed by 4 booster immunizations with 0.5 mg TROP2 protein 1:1 equal volume mixed with incomplete Freund's adjuvant, a total of 5 immunizations, with a single interval of 20 days. Seven days after each immunization, a small amount of camel peripheral blood was drawn for titer detection. After the last detec...
Embodiment 2
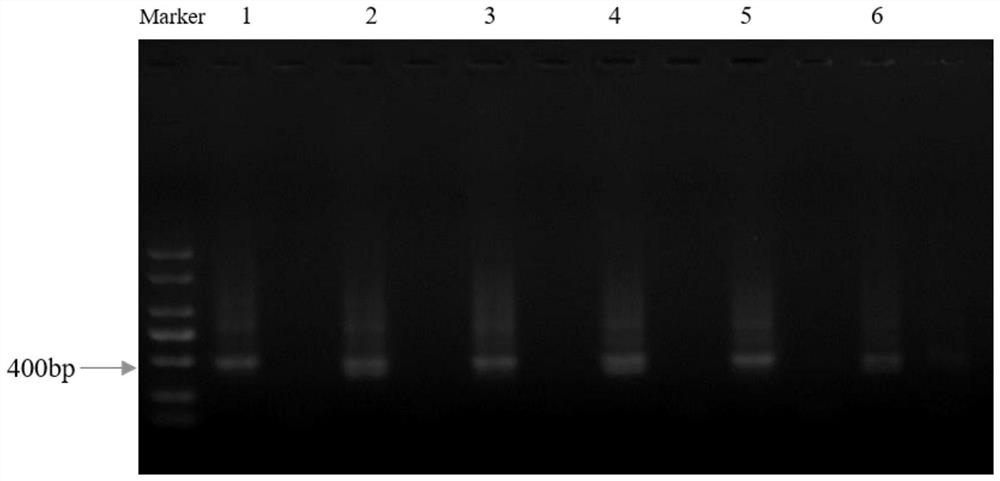
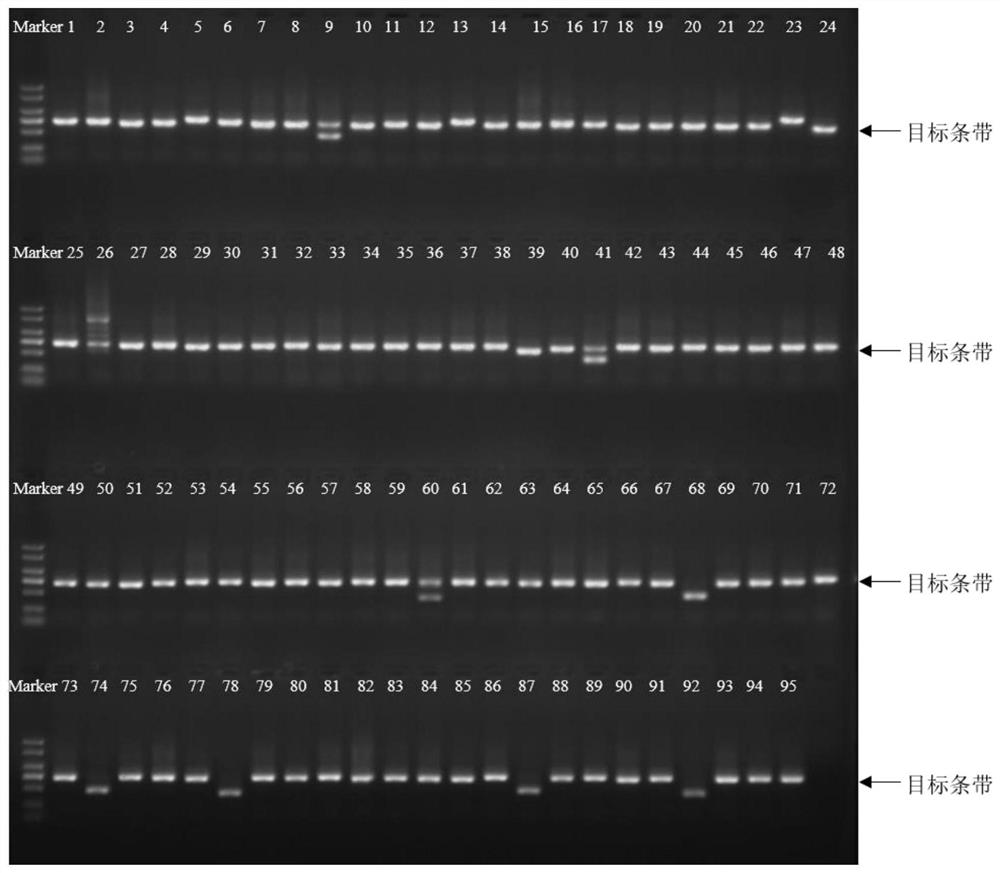
[0063] Example 2: Screening of positive clones expressing Nanobody TROP2-VHH
[0064] 2.1 Affinity panning
[0065] 制备固相蛋白ELISA板:用1x carbonate / bicarbonate buffer配制人TROP2-His抗原(公司自产,氨基酸序列为MDMRVPAQLLGLLLLWFPGSRCHTAAQDNCTCPTNKMTVCSPDGPGGRCQCRALGSGMAVDCSTLTSKCLLLKARMSAPKNARTLVRPSEHALVDNDGLYDPDCDPEGRFKARQCNQTSVCWCVNSVGVRRTDKGDLSLRCDELVRTHHILIDLRHRPTAGAFNHSDLDAELRRLFRERYRLHPKFVAAVHYEQPTIQIELRQNTSQKAAGDVDIGDAAYYFERDIKGESLFQGRGGLDLRVRGEPLQVERTLIYYLDEIPPKFSMKRLTHHHHHHHHHH),使其终浓度为1μg / ml,以100μl / 孔加液于96孔酶标 Plate (temporarily divided into three areas A, B, and C, each area has 4 wells), overnight at 4°C. After coating, the plate was washed once with PBS solution containing 0.05% Tween 20 (ie 1xPBST), then 200 μl / well of PBST solution containing 2% BSA was added, and placed at 37°C for 2 hours to block. Phage blocking: Add 20 μl of purified Phage and 16 μl of human immunoglobulin IgG (pH4) for intravenous injection (purchased from Taibang, Guizhou) to 364 μl of PBST buffer containing 5% skim...
Embodiment 3
[0076] Example 3: In Vitro Assay for Assaying Anti-TROP2 Nanobody Functional Activity
[0077] 3.1 Reference antibody blocking ELISA
[0078] The clone TROP2-VHH-01-9F and the clone TROP2-VHH-01-5A were expanded and cultivated at 37° C. at 200 rpm until the OD600 value was 0.6-0.8. Add final concentration of 1mM IPTG, induce overnight at 30°C, 150rpm. The cultured cells were ultrasonically disrupted, and then purified using BeaverBeads IDA-Nickel (Beaver Bio, Cat#70501-100) to obtain anti-TROP2 nanobody.
[0079] The blocking ability of anti-TROP2 Nanobodies to block reference antibody / TROP2 antigen binding was evaluated by competition ELISA method. The brief description is as follows, the reference antibody (Benchmark1) was prepared with 1x carbonate / bicarbonate buffer to a final concentration of 1 μg / ml, added to a 96-well ELISA plate at 100 μl / well, and coated overnight at 4°C. The next day, after washing the plate once with PBST, add 200 μl / well of PBST solution contain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com