Synthesis method of 3-aminopyridine formaldehyde
A technology of aminopyridinecarboxaldehyde and synthesis method, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of high toxicity of reaction reagents, expensive catalysts, troublesome post-processing, etc., and achieves convenient operation and post-processing, easy to enlarge, and low cost of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further described below in conjunction with specific examples, but the examples are only exemplary and do not constitute any limitation to the scope of the present invention. Those skilled in the art should understand that the details and forms of the technical solutions of the present invention can be modified or replaced without departing from the spirit and scope of the present invention, but these modifications and replacements all fall within the protection scope of the present invention.
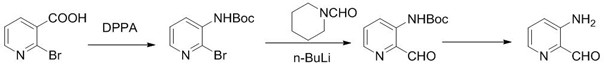
[0025] In the present invention, the synthetic technique of 3-aminopyridine formaldehyde is to use 2-bromonicotinic acid as raw material to carry out curtius rearrangement reaction to obtain (2-bromopyridin-3-yl) tert-butyl carbamate, and carry out Grignard reaction to obtain (2-methyl Acylpyridin-3-yl) tert-butyl carbamate, and finally hydrolyzed to give 3-aminopyridinecarbaldehyde. The reaction formula is as follows:
[0026] .
[0027] The sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com