A uveal melanoma metastasis risk prediction kit
A technology for melanoma and uvea, applied in the field of disease molecular diagnosis kits, can solve problems such as unclear mechanism and relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 PCR detection and sequencing kit composition of the present invention
[0033] The kit of the invention includes amplification reagents for amplifying the p.E725K mutation site of the EZH2 gene, and reagents for Sanger sequencing.
[0034] 1. Amplification reagent
[0035] The PCR amplification reagent is used to amplify a DNA sequence where the SNP site is located, and its composition is shown in Table 1.
[0036] Table 1 PCR amplification reagents
[0037] component concentration volume PCR mix 2× 600μl primer pair 10μM 100μl pure water 2ml
[0038] The PCR mixture in Table 1 includes Taq enzyme, dNTP, magnesium ions and other components required for conventional PCR; the primer pair information is shown in Table 2.
[0039] Table 2 Primers used for gene amplification
[0040]
[0041] 2. Sequencing reagents
[0042] The reagent included the components shown in Table 3.
[0043] Table 3 Gene variant typing...
Embodiment 2
[0047] Example 2 The method of using the kit of the present invention
[0048] 1. DNA extraction
[0049] The paraffin blocks of UM patients were extracted for sectioning, 5-10 μm thick, 5-6 slices. Use a scalpel to scrape off the tumor tissue on the section, try to scrape only the target tissue part, and remove excess paraffin. The scraped tissue was collected in a 1.5 mL EP tube, 1 mL of xylene was added, and 1 mL of xylene was added to mix well. After fully dissolving the paraffin, centrifuge at 12,000 rpm for 2 min at room temperature, and discard the supernatant. Add 1 ml of absolute ethanol, shake and mix well, centrifuge at 12,000 rpm for 2 min at room temperature, and discard the supernatant. Open the cap and dry at room temperature or 37°C for 5-10 minutes to completely remove residual ethanol. Add 200 μl of lysis buffer and 20 μl of Proteinase K, mix well, and incubate at 55°C until the sample is completely lysed. Incubate in a 90°C water bath for 1 h, add 200 μl...
experiment example 1
[0069] Experimental Example 1 The relationship between UM metastasis and p.E725K mutation
[0070] 1. Selection of experimental subjects
[0071] From 2009 to 2017, 85 patients with primary UM were collected from West China Hospital of Sichuan University. Among them, 19 had UM cell metastasis within 5 years of illness, and 66 had no UM cell metastasis within 5 years of illness. The rate was 22.35%. The specific clinical case parameters are shown in Table 7.
[0072] Table 7 Clinicopathological parameters of 85 UM patients
[0073]
[0074] 2. Sequencing
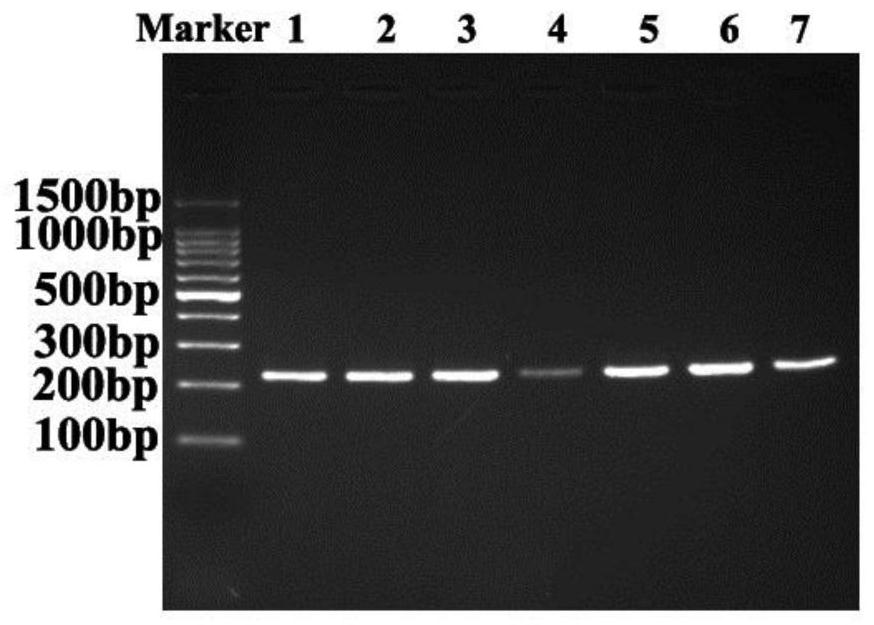
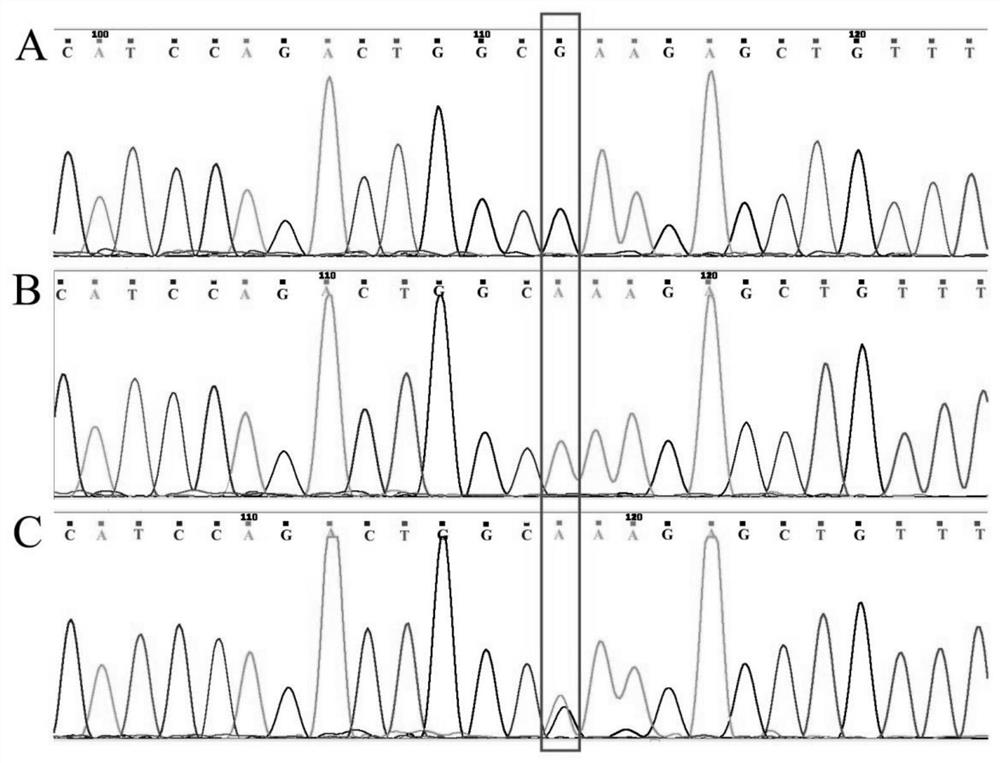
[0075] The method of Example 2 was used to sequence the p.E725K mutation site of the EZH2 gene of the experimental subject.
[0076] 3. Sequencing results
[0077] The results showed that there was a p.E725K mutation site in the EZH2 gene of the experimental subjects, and the mutation rate was 41.2% (35 / 85). Among the patients with UM cell metastasis for 5 years, 13 patients carried the p.E725K mutation (metastasis). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com