Novel cyclic dinucleotide derivative and antibody-drug conjugate thereof
An antibody drug and conjugate technology, applied in the field of antibody drug conjugates and cyclic dinucleotide derivatives, can solve the problem of no specific examples of conjugates, no confirmed anti-tumor effects of conjugates, no records Problems such as conjugate examples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
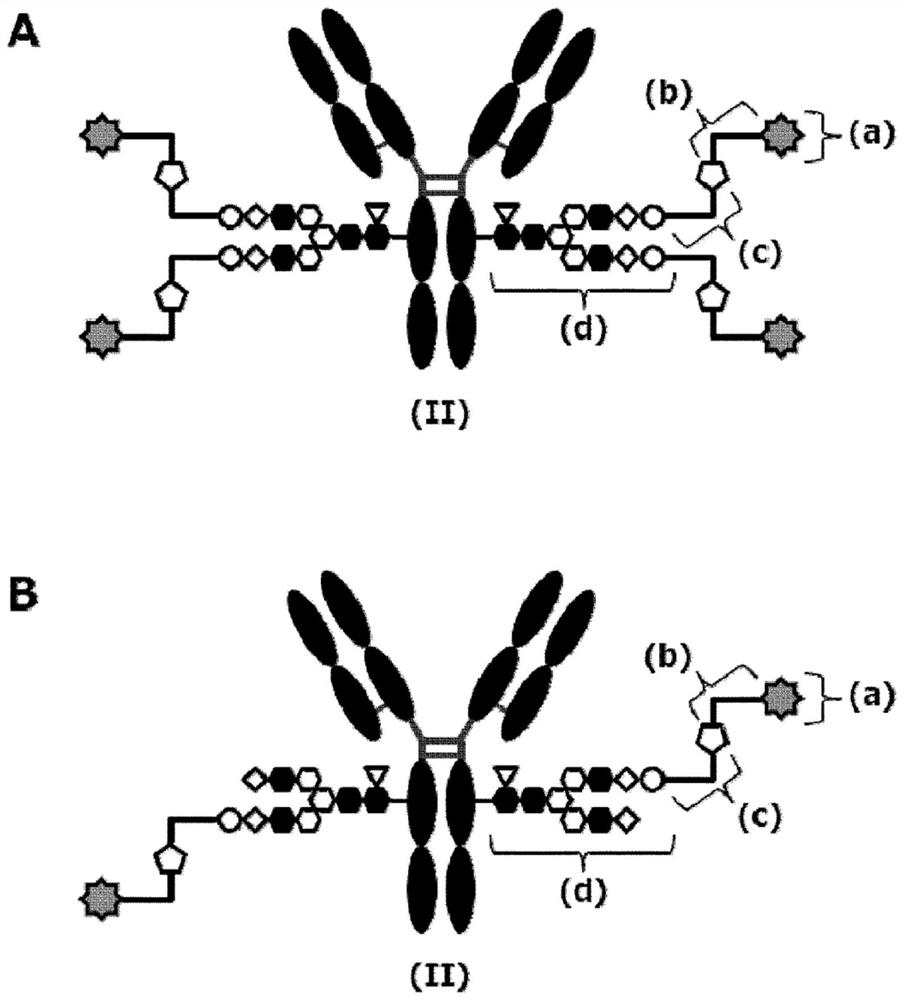
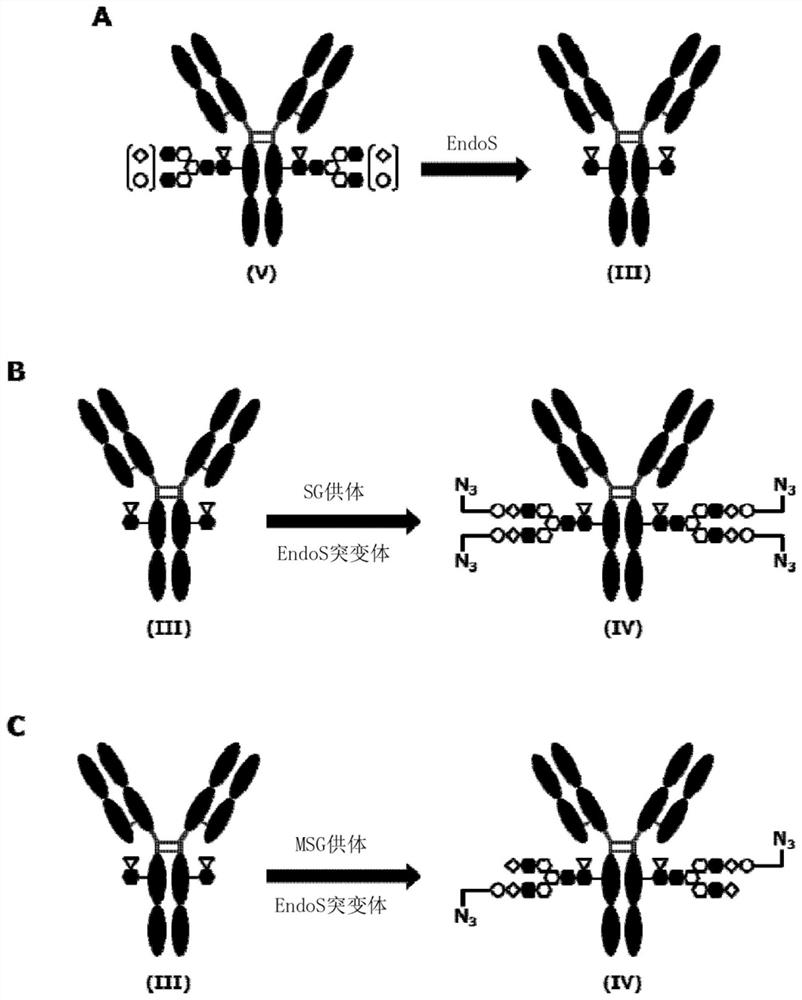
[0616] In the preparation of the above-described sugar chain-reconstituted antibody, concentration of the antibody aqueous solution, concentration measurement, and buffer exchange can be performed according to the common procedures A to C described later.
[0617] In addition, the azide sugar chain oxazoline body of SG type was synthesized according to the method described in WO2018 / 003983. As an example, [N 3 -PEG(3)] 2 The synthesis method of -SG(10)-Ox (compound 1-10 described in WO2018 / 003983) is shown in the following formula.
[0618]
[0619] The azidosugar chain oxazoline body of MSG type was also synthesized according to the method described in WO2018 / 003983. As an example, [N 3 The synthesis method of -PEG(3)]-MSG1(9)-Ox (compound 1-11 described in WO2018 / 003983) is shown in the following formula.
[0620]
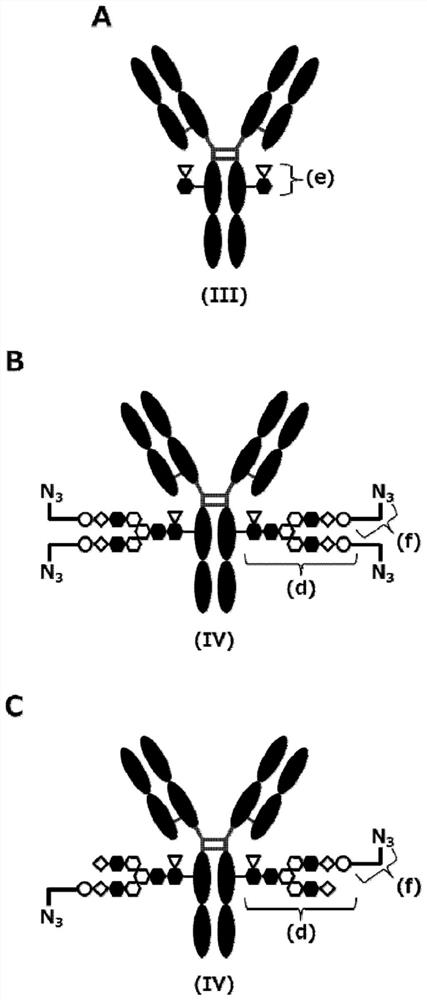
[0621] Method E: Conjugation of antibodies and drugs (sugar chain coupling 1)
[0622]
[0623] (In the formula, the two asterisks (*) on the left ...
Embodiment 1
[0732] Example 1: Synthesis of CDN1
[0733] (5R, 7R, 8R, 12aR, 14R, 15R, 15aS, 16R)-7-(6-amino-9H-purin-9-yl)-15,16-dihydroxy-2,10-bis(mercapto)- 14-(6,7,8,9-tetrahydro-2H-2,3,5,6-tetraazabenzo[cd]azulene-2-yl)octahydro-2H, 10H, 12H-5,8 - A bridge - 2λ 5 , 10λ 5 - Furo[3,2-l][1,3,6,9,11,2,10]pentoxabisphosphine cyclotetradecyne-2,10-dione
[0734]
[0735] [synthesis path]
[0736]
[0737] (Process 1)
[0738] 7-{2-O-[tert-butyl(dimethyl)silyl]-3,5-O-(di-tert-butylsilyl)-β-D-ribofuranosyl}-5- Iodo-7H-pyrrolo[2,3-d]pyrimidin-4-amine
[0739] In the N,N-dimethylformamide (10 mL) solution of 5-iodo tuberculin (1.0 g) known in the literature (Tetrahedron 2007, 63, 9850-9861), slowly dropwise at 0°C After di-tert-butylsilylbis(triflate) (1.24 mL), the mixture was stirred at the same temperature for 30 minutes. After adding imidazole (868 mg) at 0°C, the temperature was raised to room temperature and stirred for 30 minutes. At room temperature, tert-butyldimethylsil...
Embodiment 2
[0797] Example 2: Synthesis of CDN2
[0798] (5R, 7R, 8R, 12aR, 14R, 15R, 15aS, 16R)-7-(6-amino-9H-purin-9-yl)-15,16-dihydroxy-2,10-bis(mercapto)- 14-(6,7,8,9-tetrahydro-2H-2,3,5,6-tetraazabenzo[cd]azulene-2-yl)octahydro-2H, 10H, 12H-5,8 - A bridge - 2λ 5 , 10λ 5 - Furo[3,2-l][1,3,6,9,11,2,10]pentoxabisphosphine cyclotetradecyne-2,10-dione
[0799] (Diastereomer 4 of Compound 1 described in Example 1)
[0800]
[0801] [synthesis path]
[0802]
[0803] (Process 1)
[0804] N-benzoyl-3'-O-[tert-butyl(dimethyl)silyl]-2'-O-[hydroxy(oxo)-λ 5 -phosphino]adenosine
[0805] Commercially available (ChemGenes) N-benzoyl-5'-O-[bis(4-methoxyphenyl)(phenyl)methyl]-3'-O-[tert-butyl(dimethyl )silyl]-2'-O-{(2-cyanoethoxy)[bis(prop-2-yl)amino]phosphino}adenosine (962 mg) in the same manner as in Example 1, Step 7 Method The reaction was carried out to give the title compound in acetonitrile. The acetonitrile solution was used directly in the next reaction.
[0806] (Process ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap