Acid-base addition salts containing berberine, processes for their preparation and their use as medicines
An addition salt and berberine technology, applied in the field of medicine, can solve the problem of reducing the dosage of berberine, unrevealed, unrevealed beneficial effects of acid-base addition salt of fenofibric acid and berberine, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 General synthetic method of acid-base addition salt of berberine
[0027] Berberine hydrochloride (1.0 equivalent) was dissolved in hot pure water and cooled to room temperature. At the same time, dissolve the organic acid (0.6-1.2 equivalents) in absolute ethanol, and add an aqueous solution of sodium carbonate (0.6-1.2 equivalents) dropwise thereto. After the addition is completed, stir the reaction system for 30-60 minutes to obtain sodium organic acid saline solution. At a temperature of 60-80°C, add the berberine hydrochloride solution dropwise to the prepared organic acid sodium salt solution, stir for 2 hours, cool, filter and collect the solid precipitate in the reaction system, wash with a small amount of cold water, dry, and Ethanol and ethyl acetate mixed solvent carry out recrystallization to obtain the acid-base addition salt pure product of berberine, can identify the composition ratio of organic acid and berberine in the acid-base addition salt...
Embodiment 2
[0029] Example 2 Screening of lipid-lowering activity of berberine acid-base addition salt
[0030] Eight-week-old male ICR mice were adaptively fed for 5 days and randomly divided into 6 groups. The mice in the blank group and the model group were intragastrically administered with 0.5% CMC-Na solution once a day; the mice in the treatment group were intragastrically administered with the test drug once a day, both at a dose of 30 μM / kg, for 7 consecutive days. Sixteen hours before the last administration, the mice in the blank group were intraperitoneally injected with normal saline, and the mice in the other groups were injected intraperitoneally with 400 mg / kg Triton-WR1339 to establish a model. One hour after the last administration, blood was collected from the orbit, left to stand for one hour, and the serum was separated by centrifugation. The levels of TC and TG in the serum were measured. The experimental results are shown in Table 1.
[0031] Table 1: Effects of ac...
Embodiment 3
[0035] Example 3 Study on improvement of metabolic syndrome in vivo by acid-base addition salt of berberine
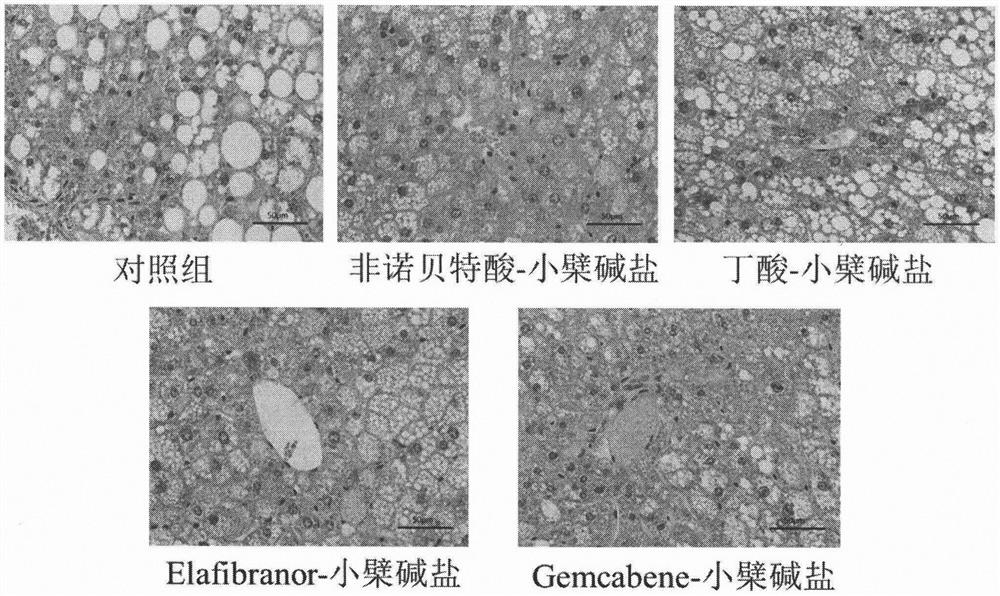
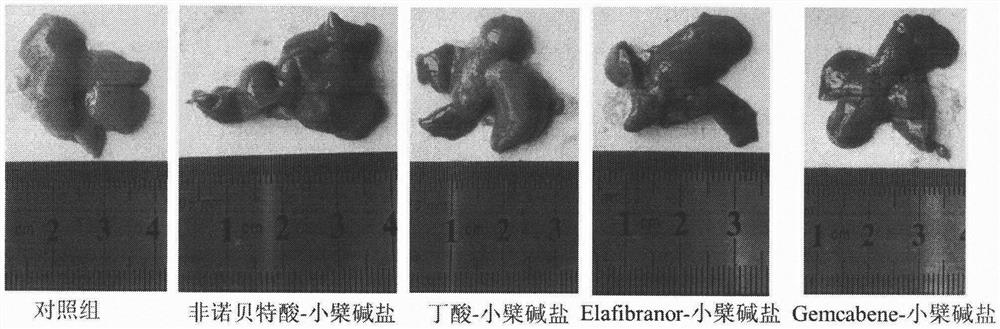
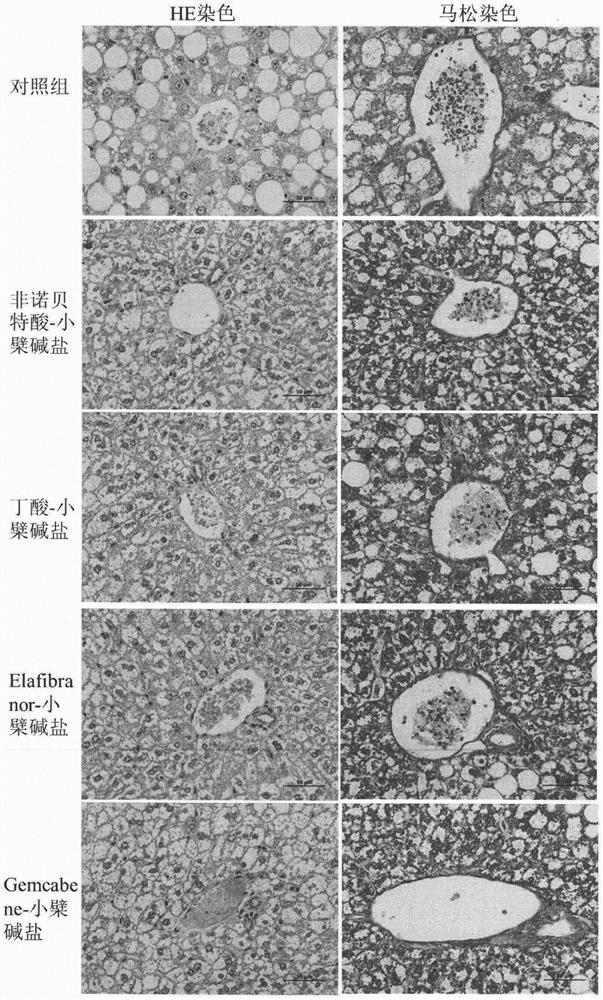
[0036] Eight-week-old ob / ob mice, male, were randomly divided into groups, 6 mice in each group, blank control group (blank vehicle: 0.5% sodium carboxymethylcellulose solution), and the test drug group was administered by intragastric administration once a day, respectively. Compound (30mg / kg), after continuous administration for 30 days, blood was collected from the mice on an empty stomach, and the levels of glycosylated hemoglobin, blood lipids, and liver function in the mice were measured with an automatic biochemical analyzer. The results are shown in Table 2. The mouse liver tissue was taken for sectioning, and the pathological changes of the liver tissue were observed by HE staining. The results are shown in the attached figure 1 .
[0037] Table 2: Effects of berberine acid-base addition salts on the levels of glycosylated hemoglobin, blood lipids, and liver ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com