Dammarane type tetracyclic triterpenoids and extraction method and application thereof
A dammarane-type, tetracyclic triterpene technology, which is applied in the field of Cyclocarya paliurus extract, can solve the problems of activity and mechanism research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
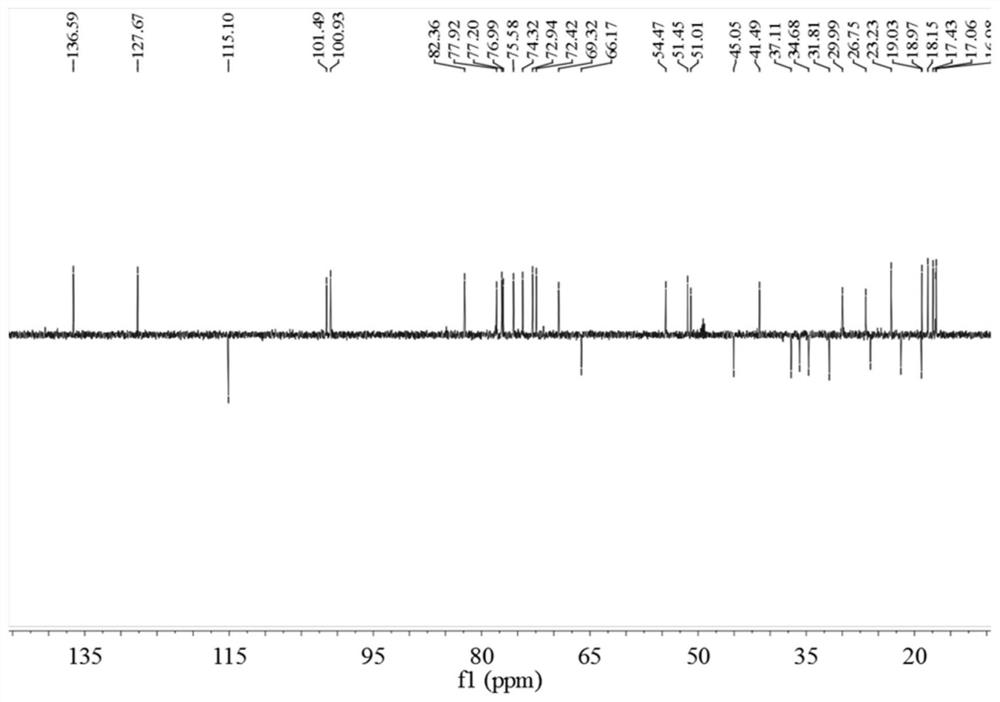
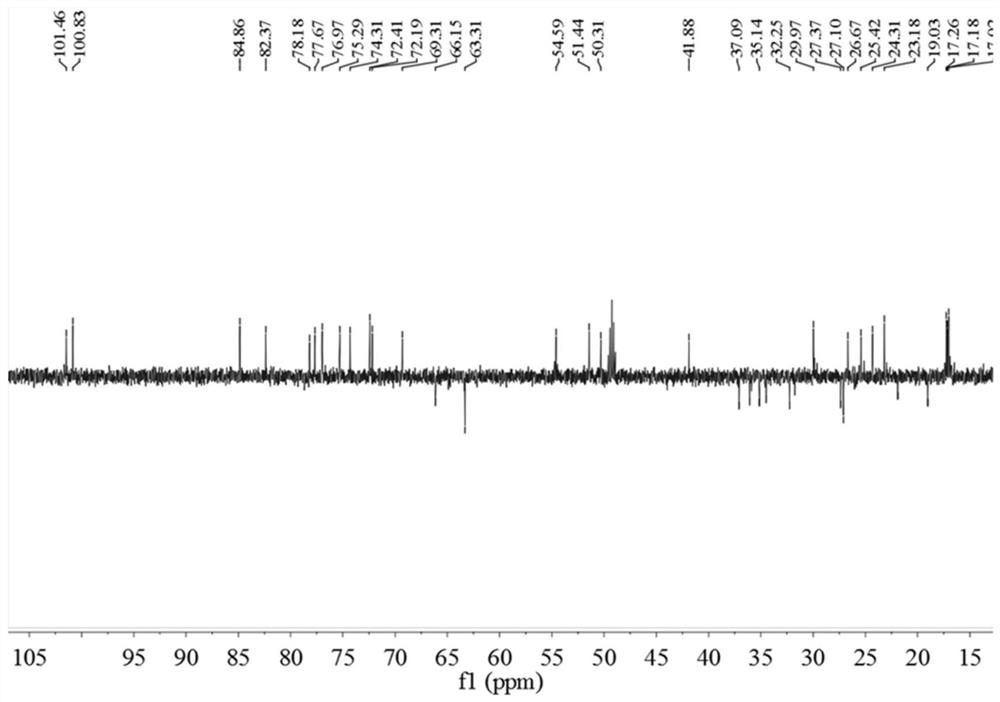
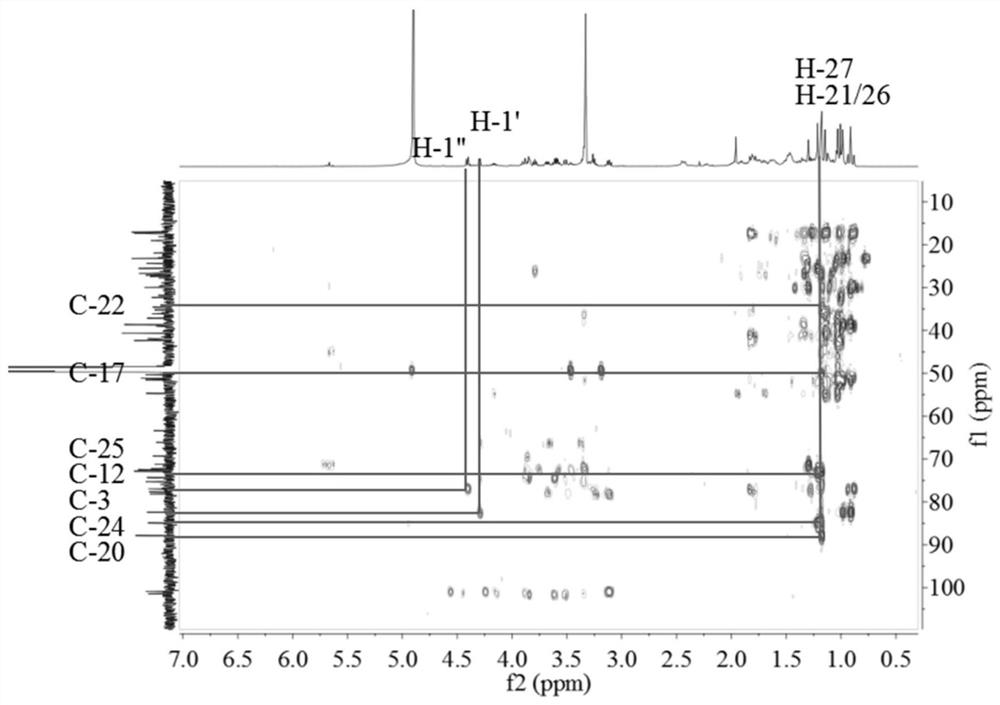
[0048] 1.1 Treatment of Cyclocarya paliurus medicinal materials: Dry leaves of Cyclocarya paliurus (10Kg), crush and extract with 70% ethanol under reflux at 120°C (100L; 2×2h), concentrate at 60°C to obtain extract, and the concentration time is 2 days. The extract was further dispersed in water, extracted with dichloromethane, ethyl acetate, and n-butanol in sequence, 10 L each time, extracted 3 times, and the extract was concentrated at 60°C for 24 hours to obtain extracts and concentrates of different polar parts. liquid. The dichloromethane part is mainly dammarane-type tetracyclic triterpenoids (marked as QQL-ST).
[0049] 1.2 Biological activity-oriented screening of active parts of Cyclocarya paliurus: Using adjuvant arthritis rat animal model to track and screen active parts of Cyclocarya paliurus for improving rheumatoid arthritis:
[0050] 1.3 Research on active site components
[0051] 1.3.1 Extraction, separation and purification of triterpenes: Use macroporous ...
Embodiment 2
[0091] Application of QQL-ST and dammarane-type tetracyclic triterpenoids in the preparation of drugs for treating rheumatoid arthritis
[0092] 1. Bioactivity-oriented screening of active sites:
[0093] Anti-RA effect experiment of adjuvant rat model Several SD rats, male, weighing 180-220g, 10 were taken as the normal control group, and the rest were used as the model control group. The rats in the model control group were injected subcutaneously at the right hind foot plantar Freund's complete adjuvant 0.1mL / body, 7 days after modeling, the toe volume meter was used to measure the degree of swelling of the toes, and according to the degree of swelling of the toes, they were randomly divided into model control group, positive control group, and QQL-ST group, each with 6 Animals, the dosage is 150mL / kg. Before daily administration, each compound was formulated with 0.5% CMC-Na to make a suspension of corresponding concentration, and the rats in each group were given the sus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com