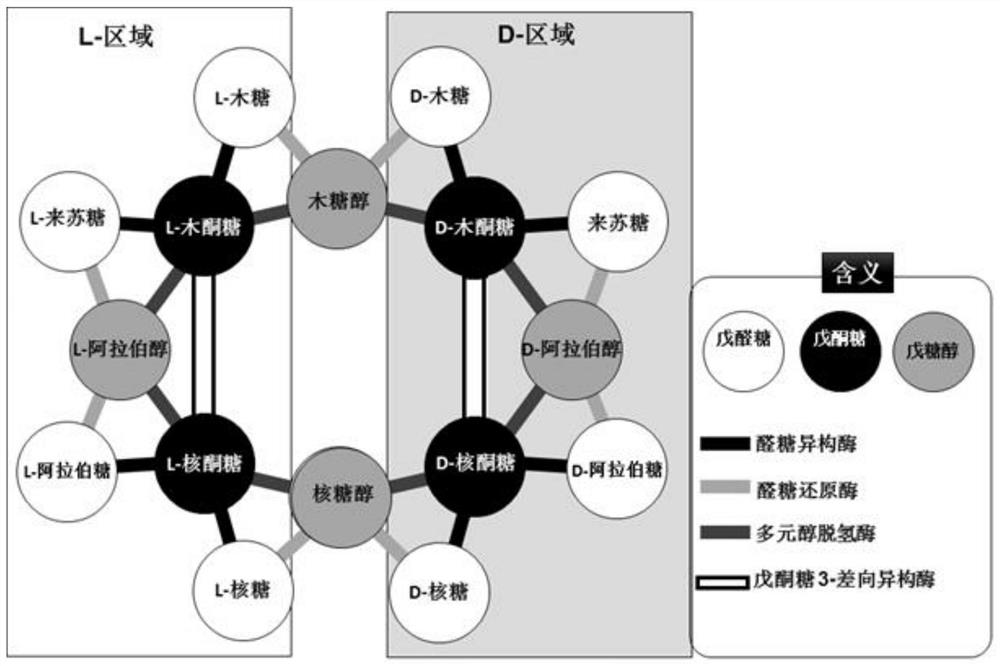
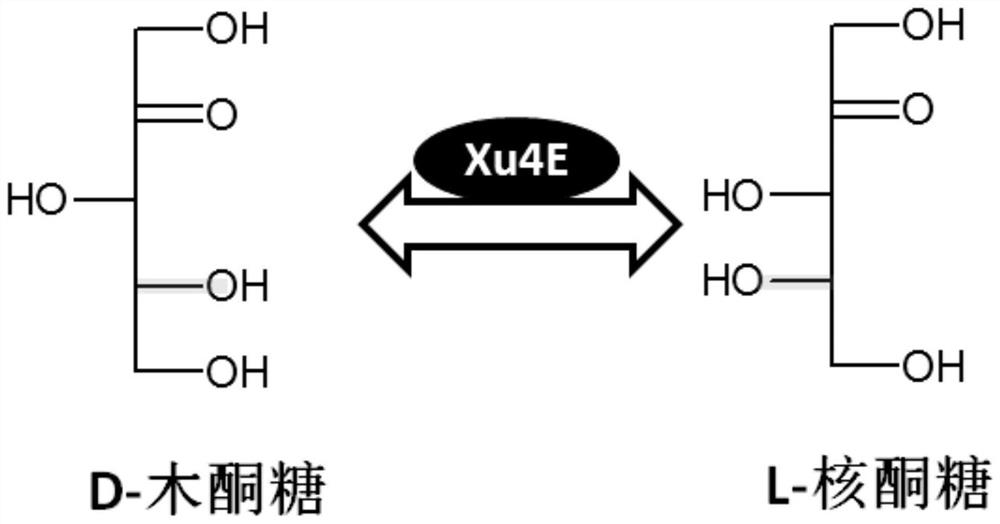
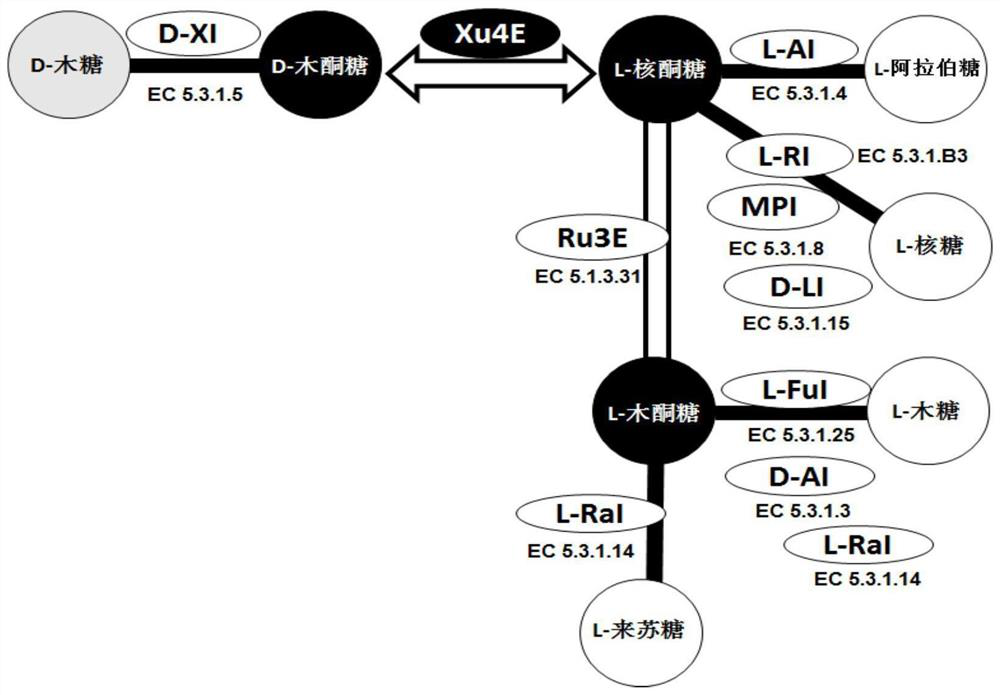
D-xylulose 4-epimerase, mutant and application thereof
A technology of epimerase and xylulose, applied in the field of D-xylulose 4-epimerase, can solve the problems of high production cost and complex production process of L-pentose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0332] Example 1. Separation and detection of pentose sugars
[0333] D-xylose, D-xylulose, D-ribulose and L-arabinose are separated by using any of the methods described in (1)-(4) below:
[0334] (1) Bio-Rad (Bio-Rad) Aminex HPLC HPX-87H liquid-phase ion exchange column was used for separation, and the separation conditions were as follows: the column temperature was 60° C., the mobile phase was 5 mM sulfuric acid, and the flow rate was 0.6 mL / min.
[0335] (2) Bole Aminex HPX-87P lead ion exchange column, the column temperature is 80℃, the mobile phase is deionized water, and the flow rate is 0.6mL / min;
[0336] (3) Waters Sugar Pak I calcium ion exchange column, the column temperature is 80°C, the mobile phase is deionized water, and the flow rate is 0.5mL / min; and
[0337] (4) Shodex Sugar KS-801 sodium ion exchange column, the column temperature is 70°C, the mobile phase is deionized water, and the flow rate is 0.5 mL / min.
[0338] For D-xylose, D-xylulose, D-ribulose,...
Embodiment 2
[0340] Example 2. Mining of enzymes with Xu4E function from L-ribulose-5-phosphate 4-epimerase
[0341] Considering the similarity of the substrate structure and the possible enzymatic catalytic mechanism, we selected from the L-ribulose-5-phosphate 4-epimerase family (RP4E, EC 5.1. Conversion of xylulose to L-ribulose functional Xu4E enzyme. We cloned four RP4Es from Bacillus subtilis 168, Geobacillus stearothermophilus, Escherichia coli, and T. maritima, respectively, and cloned them to the pET plasmid. E. coliBL21 (DE3) carrying the expression plasmid was grown and the recombinant protein was expressed.
[0342] After the protein with His-tag was purified by affinity adsorption, the protein with His-tag was purified by the method described in this disclosure for " Judgment more Determination of whether a peptide / enzyme has Xu4E enzymatic activity”, and tested the obtained recombinant protein. From the test results, the three RP4Es derived from Thermus marinus, Bacillu...
Embodiment 3
[0345] Example 3. Mining of new enzymes with Xu4E function from tagatonic acid 3-epimerase
[0346] Considering the similarity of the substrate structure and the possible enzymatic catalysis mechanism, we adopted a method similar to Example 2 to select a possible enzyme from tagatonic acid 3-epimerase (UxaE, EC 5.1.2.7). A new enzyme capable of converting D-xylulose to L-ribulose. We cloned multiple uxae genes from different microorganisms and cloned them into pET plasmids. E. coli BL21 (DE3) carrying the expression plasmid was grown and the recombinant protein was expressed.
[0347] After the protein with His-tag was purified by affinity adsorption, the protein with His-tag was purified by the method described in this disclosure for " Judgment more Determination of whether a peptide / enzyme has Xu4E enzymatic activity ”, to detect the obtained recombinant protein. From the detection results, the tagatonic acid 3-epimerase (Tm0440) from Thermus marinus has no optimized re...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap