Low-intensity treatment of hematological disorders
A blood and leukemia technology, applied in the direction of antibody medical ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as low effective rate and depression of single-drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 17
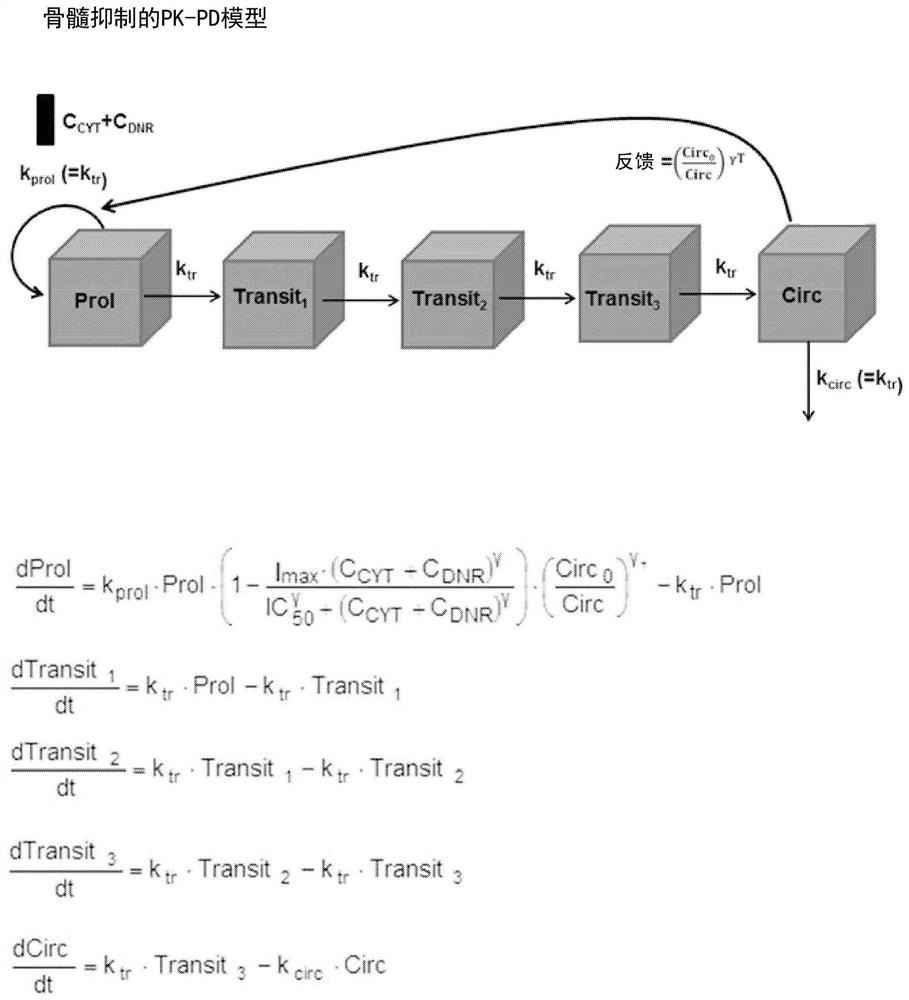
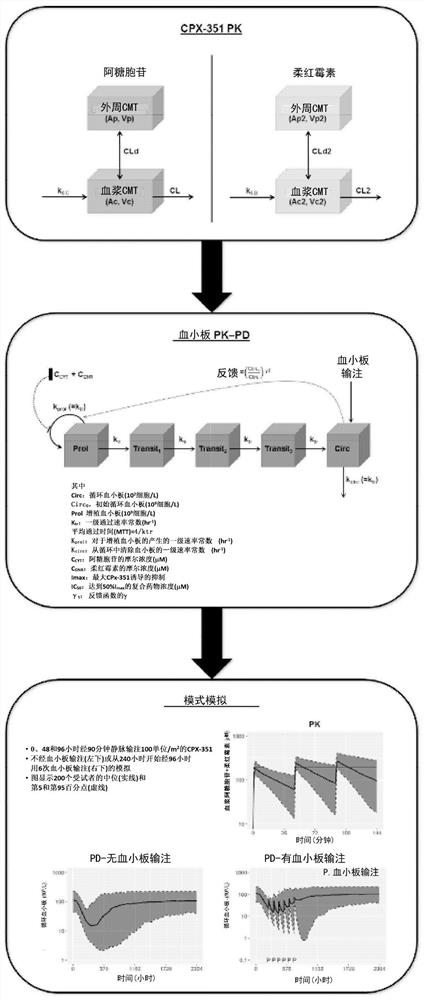
[0082] Example 17+3 or CPX-351-induced population pharmacokinetics-pharmacodynamics (PK-PD) model of thrombocytopenia
[0083] The following CPX-351 studies were used for PK-PD modeling:
[0084] Research 101:
[0085] Phase 1 open-label dose-escalation study of CPX-351 in 48 adult patients with relapsed / refractory AML, ALL or MDS
[0086] CPX-351 dose: 3 to 134 units / m by 90-minute intravenous (IV) infusion on days 1, 3, and 5 2 (1 unit = 1 mg cytarabine and 0.44 mg daunorubicin)
[0087] PK data collection: intensive sampling on days 1, 3 and 5
[0088] PD data collection: Pre-dose; Days 1, 2, 3, 4, 5, 7, 14, 21, 28, 35, 42, and 56; and Follow-up (30 days after study discontinuation)
[0089] Research 206:
[0090] A phase 2 open-label study of CPX-351 in 26 adult patients with relapsed / refractory AML, ALL or MDS
[0091] CPX-351 dosage:
[0092] o Induction: Day 1, Day 3, and Day 5 (Second Induction: Day 1 and Day 3) IV infusion of 100 units / m 2 (Cytarabine 100mg / ...
Embodiment 2
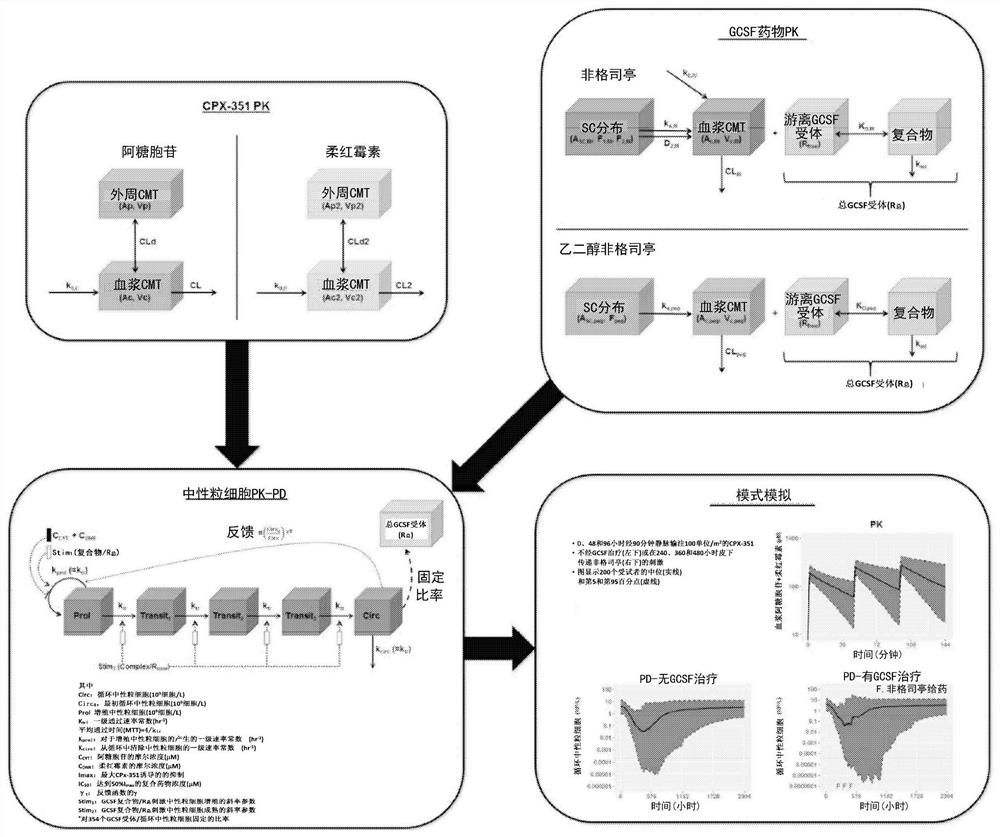
[0134] Chemotherapy-induced neutropenia (CIN) model in patients treated with embodiment 2 CPX-351 and "7+3" the establishment of
[0135] CPX-351 and 7+3 studies and cytarabine / daunorubicin modeling are shown in Example 1.
[0136] The results are displayed as follows:
[0137] Table 4. Population PK parameters for GCSF agents
[0138]
[0139] Table 4. Population PK parameters for GCSF agents
[0140]
[0141] PK, pharmacokinetics; GCSF, granulocyte colony-stimulating factor; F 1 , the bioavailability of the primary absorption process; k a , the first-order absorption rate constant; F 2 , the bioavailability of the zero-order absorption process; D 2,fil , the duration of the zero-order absorption process; K D , the equilibrium dissociation constant of GCSF and GCSF receptor; CV, coefficient of variation; CL, clearance rate; WTKG, body weight (unit: kg); V c , distribution central volume; k int , First-order rate constants for the internalization of GCSF rece...
Embodiment 3
[0161] Simulation of alternative dosing regimens for CPX-351
[0162] Myelosuppression Curve Simulation for Low Dose / Regimen:
[0163] Days 1+5; various doses 2
[0164] Days 1+8; various doses 2
[0165] Days 1+8+15; various doses 2
[0166] Other regimens; various doses 2
[0167] Cell cycle timing and Vyxeos treatment effects were further examined using the PK / PD model described in Examples 1 and 2.
[0168] Simulation results for absolute neutrophil count (ANC): Simulations were performed using a previously developed mechanistic PK / PD model for absolute neutrophil count. Figure 8 Periods of ANC below 500 / uL following CPX-351 administration are shown. The duration of ANC<500 / uL prolongs with increasing dose. Depending on the treatment regimen, the duration of the administered dose may vary. Also shown is the duration of ANC suppression following Vyxeos standard day 1, day 3 and day 5 regimen or 7+3 treatment, where Vyxeos showed a longer duration of ANC suppressio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com