Modified oncolytic virus, composition and use thereof
A technology of oncolytic virus and viral genome, applied in the field of modified oncolytic virus, can solve the problem of insufficient treatment of primary or metastatic tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0210] The following examples are set forth to aid in the understanding of the present disclosure and are not to be construed in any way as limiting the scope of the invention as defined in the claims that follow.
example 1
[0211] Example 1: Virus Construction
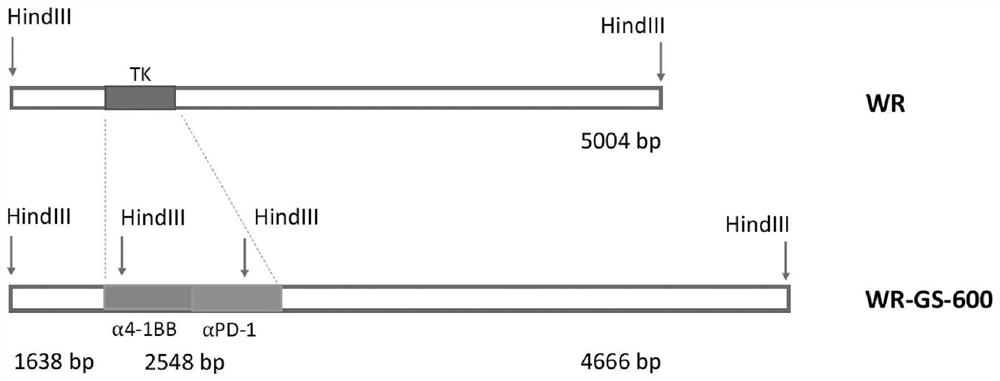
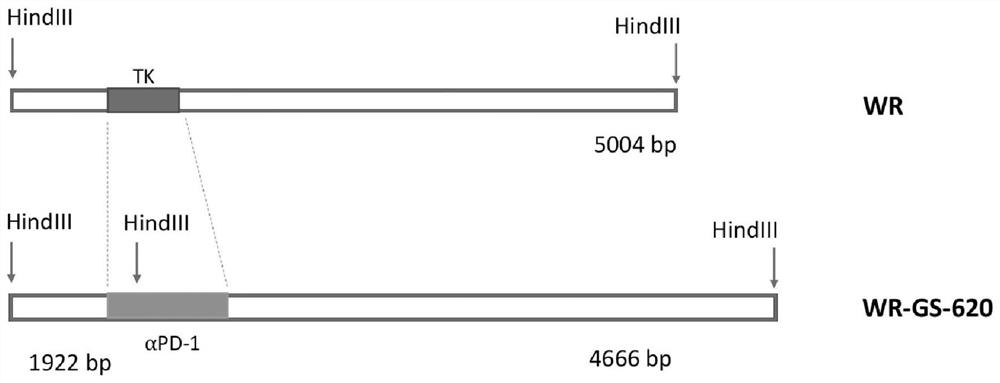
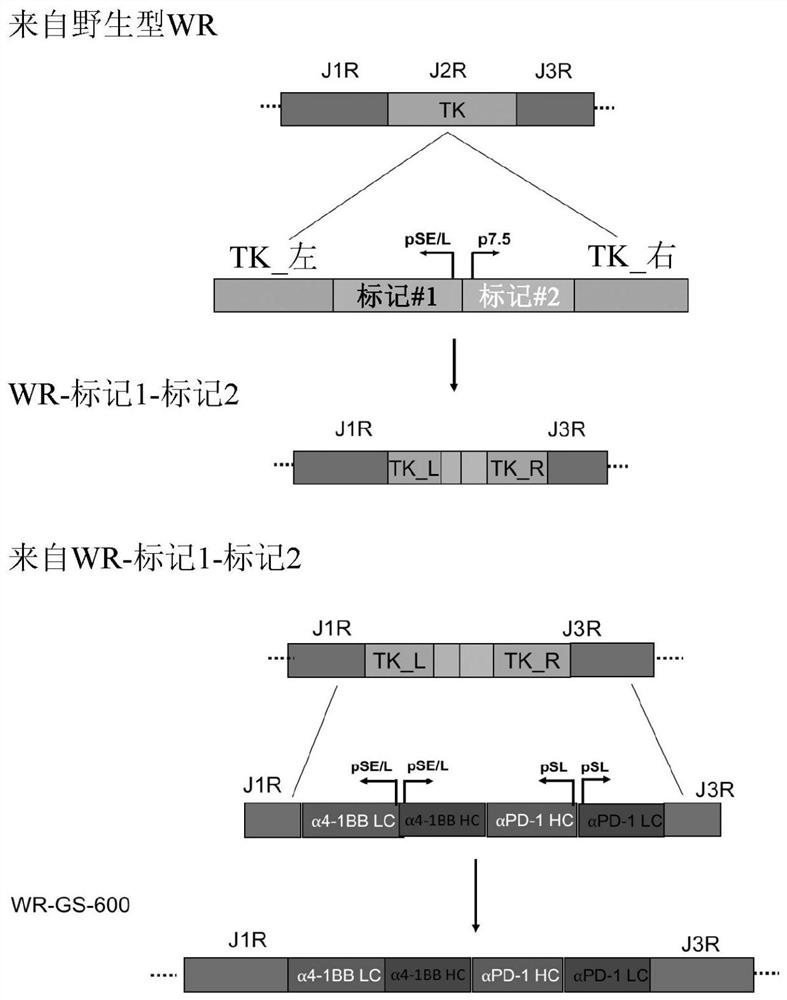
[0212] The starting WR strain of vaccinia virus was obtained from the ATCC (www.atcc.org: VR-1354). Since multiple genes are involved, WR-GS-600 was built with a step-by-step engineering approach. Briefly, in the first step, the WR DNA was recombined with the modified pSEM-1 vector (Rintoul et al., 2011) to insert the marker / selection gene into the TK locus. This allows for easy differentiation from the wild type parent for further engineering. Afterwards, the amino acid sequence and the nucleic acid sequence encoding anti-human PD-1, which have flanking sequences of J1R and J3R and encode anti-human PD-1, are shown in Figure 15 and 16 Middle) and anti-human 4-1BB (the amino acid sequence of anti-human 4-1BB and the nucleic acid sequence encoding anti-human 4-1BB are shown in Figure 17 and 18 Middle) recombinant plasmids were transfected into WR-infected U2OS cells to completely delete TK and insert antibody sequences. figure 1 st...
example 2
[0220] Example 2: Characterization of WR-GS-600, WR-GS-610 and WR-GS-620
[0221] During the engineering process of these new viruses, their genome integrity and protein expression were closely monitored.
[0222] PCR, sequencing, and restriction digests
[0223] Genomic DNA of the virus was isolated from a sucrose pad purified by treatment of the virus preparation with the totipotent nuclease endonuclease, by sucrose precipitation, followed by proteinase K and detergent treatment, followed by phenol / chloroform / isoamyl alcohol extraction and ethanol precipitation. Extract and recover DNA.
[0224] To ensure that the viral genome has the expected sequence carrying the designed antibody sequence, a series of primers have been designed, including primers within the recombination region and primers outside the engineered segment. Virus (WR-GS-600, WR-GS-610 and WR-GS-620) identities were confirmed by qPCR (TaqMan). Primers used in PCR are shown in Table 2. The locations of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com