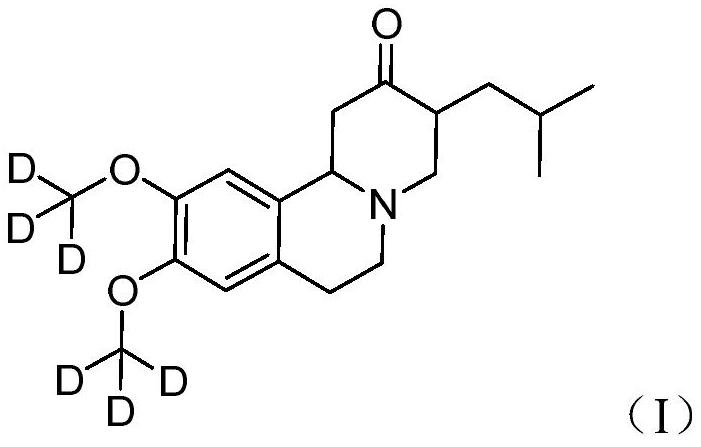
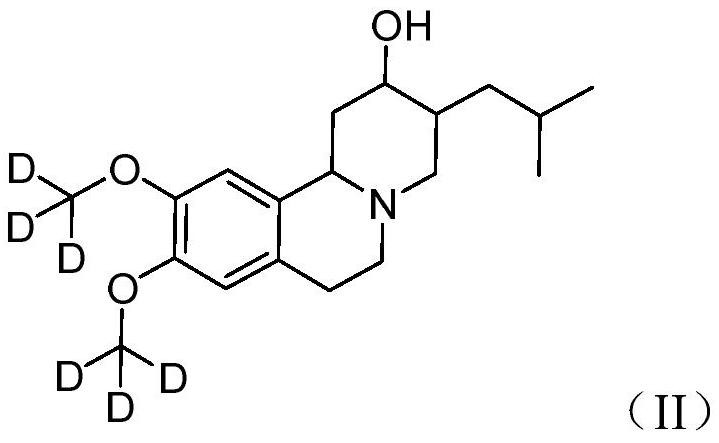
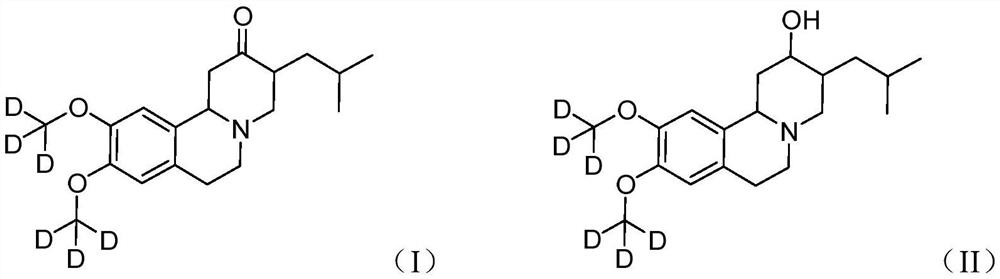
Deutetrabenazine for the treatment of dyskinesia in cerebral palsy
A movement disorder, cerebral palsy technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, nervous system diseases, etc., can solve problems such as failure to prove patient function improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0227] Example 1: Efficacy Study
[0228] A phase 3, 21-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of deutetrabenazine was conducted as follows.
[0229] Approximately 185 patients were randomized in a 2:1 ratio to deutetrabenazine versus placebo (approximately 124 in the deutetrabenazine group; approximately 61 in the placebo group), based on Stratified by age (6 to <12 years; 12 to 18 years, inclusive) and region (United States [US]; non-US). Sample size was reestimated at interim analysis (IA) and adjustments could be made for up to a total of approximately 230 patients.
[0230] The study population consisted of male and female patients diagnosed with DCP aged 6 to 18 years (inclusive).
[0231] A patient can be included in this study only if he or she meets all of the following criteria:
[0232] 1. Patients were 6 to 18 years old (inclusive) at baseline.
[0233] 2. The patient weighed at ...
example 2
[0390] Example 2: Open Label Extension Study
[0391] A Phase 3, 55-week, open-label, single-arm, long-term safety, tolerability, and efficacy study of deutetrabenazine for the treatment of movement disorders in children and adolescents with cerebral palsy was conducted as follows:
[0392] General study design: Phase 3, 21-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study ("parent study") to evaluate the efficacy and safety of deutetrabenazine has been successfully completed Patients may be eligible to participate in the study after completing a 1-week washout period and a final assessment at week 16. The study will include children and adolescents who are between the ages of 6 and 18 when they participate in the maternal study.
[0393] Screening: Screening assessments for this open-label study will be performed as part of baseline follow-up. Any adverse events initiated after the end of the maternal study and recorded after informed con...
example 3
[0440] Example 3: Efficacy Study in Adults
[0441] A phase 3 study was conducted to evaluate the efficacy and safety of deutetrabenazine in a cohort of male and female patients diagnosed with DCP. A patient can be included in this study only if he or she meets all of the following criteria:
[0442] 1. The patient is older than 18 years at baseline.
[0443] 2. The patient has symptoms of cerebral palsy (CP) from infancy (≤2 years).
[0444] 3. According to the European cerebral palsy surveillance standards, the patient was diagnosed as DCP.
[0445] 4. Based on the investigator's score of chorea, the patient's total score on the MD-CRS Part II item at the baseline visit is ≥10.
[0446] 5. The patient's symptoms lead to functional problems as determined by a Clinical Global Impression of Severity (CGI-S) score of 4 or higher based on the investigator score.
[0447] 6. Chorea is the major movement disorder as assessed by EAB at Screening.
[0448] Patients will receive ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com