Analogs of deutetrabenazine, their preparation and use
A technology of deuterated tetrabenazine and compounds, applied in the field of analogs of deuterated tetrabenazine, its preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0174] Example 1 prepares compound 1
[0175]
[0176] The crude material (10 g) obtained from the mother liquor of deuterated tetrabenazine was purified by recrystallization from EtOH (2 x 3V) to obtain 2.5 g of compound 1 as an off-white solid.
[0177] NMR Consistency Analysis of Compound 1
[0178] Compound 1:
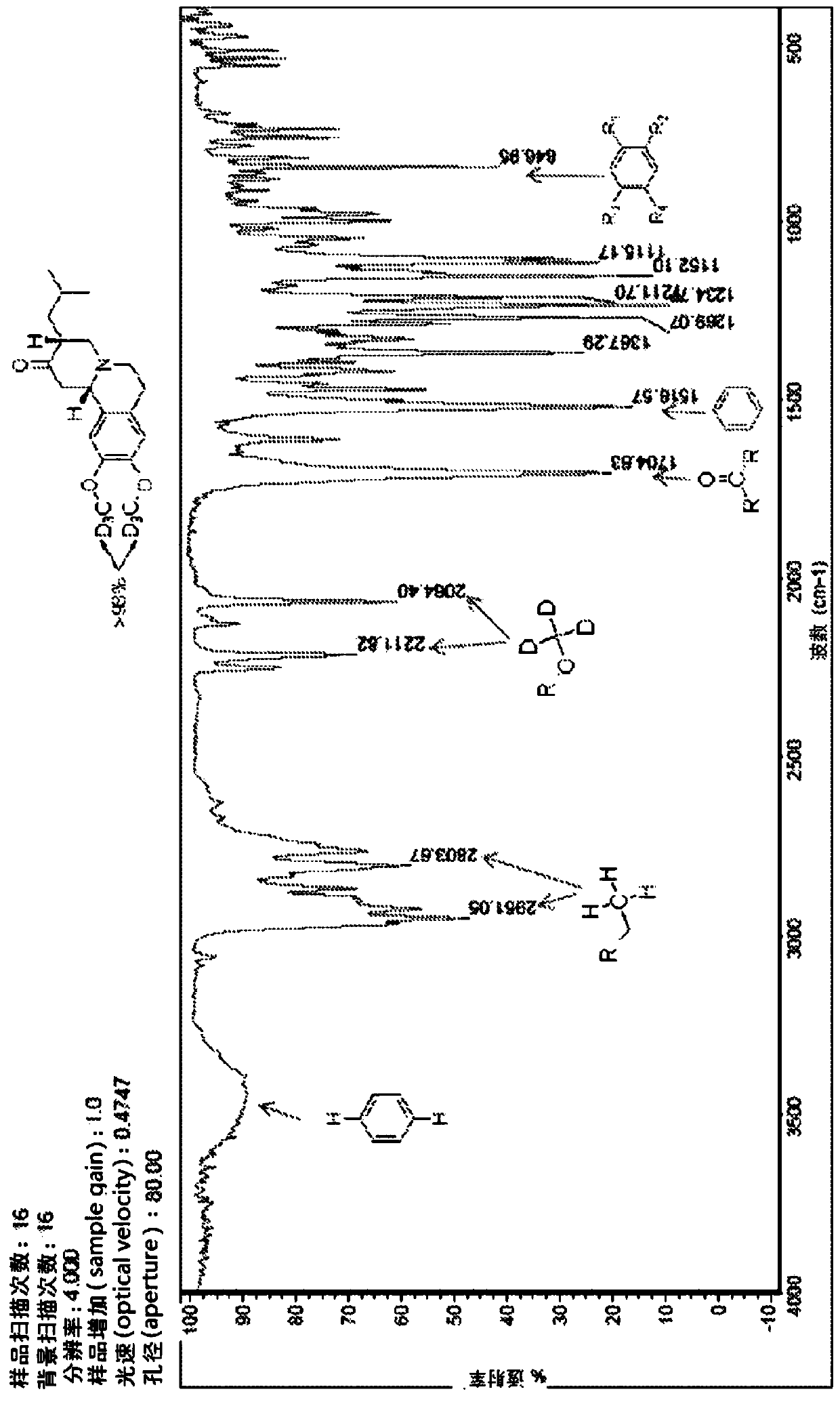
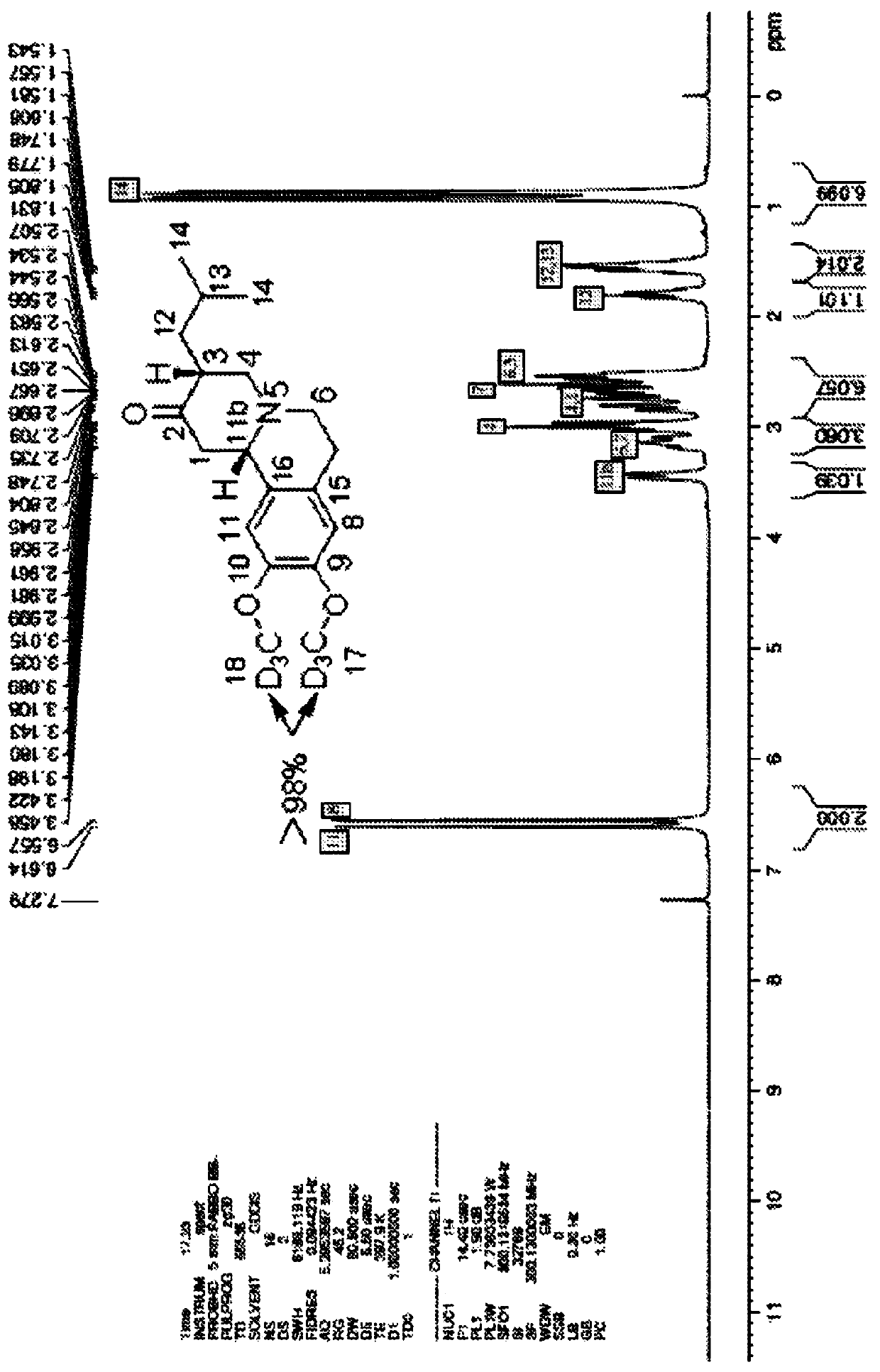
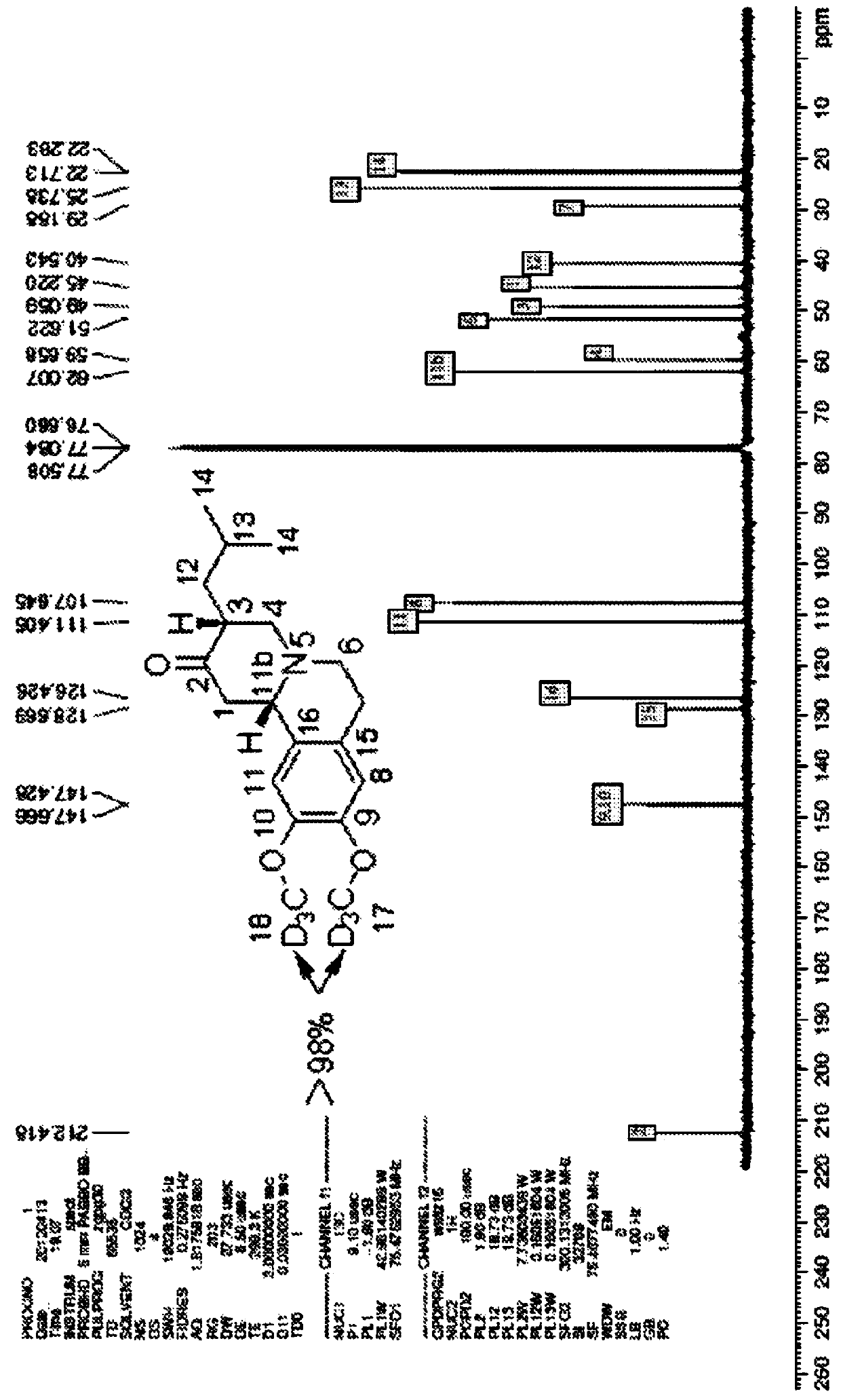
[0179] Use 300MHz NMR instrument, use compound 1 in CDCl 3 (99.9% D atoms) to determine the following data in Table 2. See Figure 2 and Figure 3.
[0180] Table 2: 1 H NMR and 13 C NMR a,c distribution of
[0181]
[0182] Location 1 H chemical shift (split mode, coupling constant)
Location 1 H chemical shift (split mode, coupling constant)
1 2.61(m,1H),2.84(m,1H), 11 6.61(s,1H) 2 N / A 11b 3.42(d,J=10.8Hz,1H) 3 2.53(m,1H) 12 1.54(m,1H),1.80(m,1H) 4 2.71(m,1H),2.98(m,1H) 13 1.55(m,1H) 5 N / A 14 0.92(m,6H) 6 2.53(m,2H),3.02(m,2H) 15 N / A 7 3.14(m,1H),2.65(m,1H) 16 N / A ...
example 2
[0186] Example 2 prepares compound 2
[0187]
[0188] To deutetrabenazine (31.7 g, 10.0 mmol) and chloranil (26 g, 10.5 mmol) was added toluene (300 mL), and the mixture was heated at reflux for 2.5 h. Add toluene (500mL) to the dark solution, and wash with 300mL of 2N NaOH and H 2 O wash mixture. The toluene solution was dried (Na 2 SO 4 ), filtered and evaporated to dryness. The residue was crystallized from ethyl acetate to obtain 21 g (66%) of compound 2 as an off-white crystalline solid.
[0189] NMR Consistency Analysis of Compound 2
[0190] Compound 2:
[0191] Use 300MHz NMR instrument to use compound 2 in CDCl 3 (99.9% D atoms) to determine the following data in Table 3.
[0192] table 3: 1 H NMR and 13 C NMR a,b distribution of
[0193]
[0194]
[0195]
[0196]
[0197] a Assignments are based on the coupling mode, coupling constant, and chemical shift of the signal.
[0198] b Spectra were calibrated with reference to NMR solvent pe...
example 3
[0200] Example 3-preparation of crude deuterated tetrabenazine
[0201] step 1 : 2-acetyl-N,N,N,4-tetramethyl-1-pentanyl ammonium iodide: 3-[(dimethylamino)methyl]-5-methyl-hexan-2-one (90 g, 0.526 mol, 1.00 eq) was charged with methyl tert-butyl ether (1.35 L, 15.0 vol) and cooled at 0-10 °C. Iodomethane (171 g, 1.209 mol, 2.3 eq) was slowly added to the reaction mixture and stirred at 25-35°C for 15 hours. The reaction was warmed to 35-40°C for 2 hours. The precipitated solid was filtered under nitrogen and washed with methyl tert-butyl ether (900 mL, 10.0 vol). The crude product was further purified by slurrying in ethyl acetate (1.46 L, 10 vol) and filtered to obtain 2-acetyl-N,N,N,4-tetramethyl-1-iodide as a white solid Ammonium pentane (146 g).
[0202] step 2 : will contain d 6 The suspension of -6,7-dimethoxy-3,4-dihydroisoquinoline (hydrochloride or free base, 1.00eq) and solvent was charged with iodide 2-acetyl-N,N,N, 4-Tetramethyl-1-pentanmonium. (if usin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com