Anti-setd3 monoclonal antibody and use thereof
A monoclonal antibody and protein technology, applied in applications, anti-enzyme immunoglobulins, instruments, etc., to achieve good recognition specificity and strong recognition specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 Monoclonal antibody and preparation method thereof
[0047] 1. Cloning construction and protein prokaryotic expression
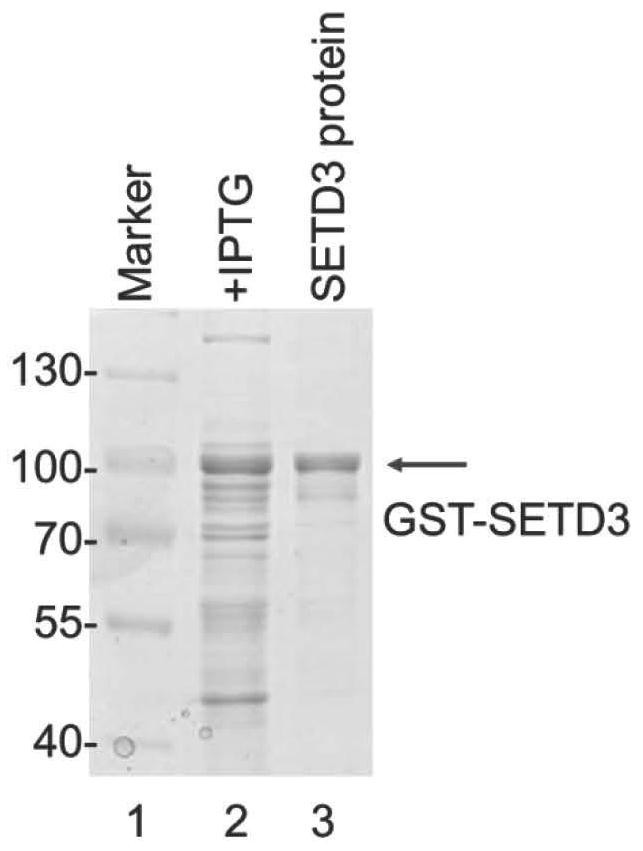
[0048] (1) Cloning the SETD3 CDS sequence into the pGEX-6p-1 vector to obtain the recombinant vector pGEX-6p-1-SETD3.
[0049] The primer pair sequences used were: forward primer (5'-ATGGGTAAGAAGAGTCGAG-3'; SEQ ID NO: 9); reverse primer (5'-TTACTCCTTAACTCCAGCAGTG-3'; SEQ ID NO: 10), cloned into pGEX-6p- 1 The EcoRI-NotI restriction site of the vector.
[0050] (2) Protein expression and purification:
[0051] ①Plasmid transformation:
[0052] The constructed SETD3 prokaryotic expression plasmid was transformed into BL21 competent cells, spread on the LB plate containing ampicillin resistance, and after the monoclonal growth, the monoclonal shake was picked.
[0053] ② Shaking bacteria & expression:
[0054] Shake the bacteria overnight, transfer a large amount the next morning, and dilute 1:100 to 1:50. Shake at 37°C to OD 600 =0.4...
Embodiment 2
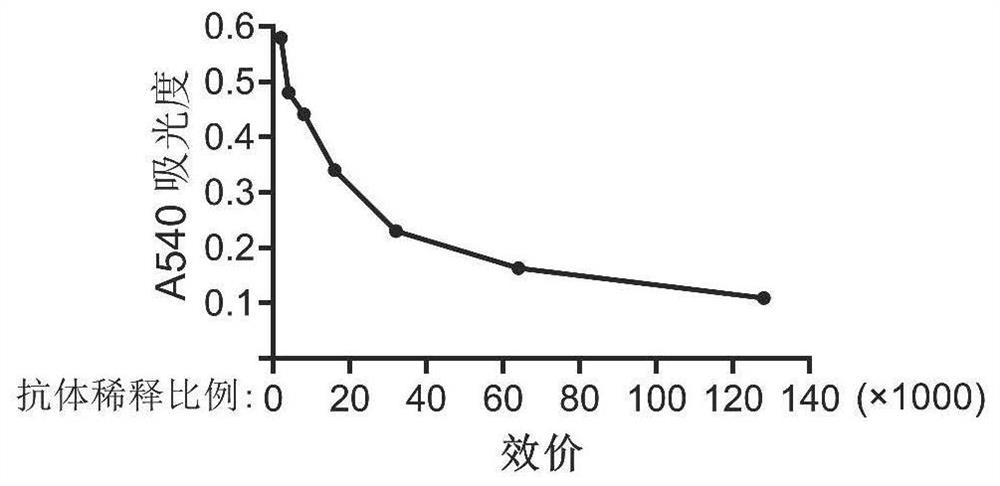
[0122] Embodiment 2, antibody titer detection
[0123] Use ELISA to determine the titer of the antibody, coat each well of the plate with 50ng of antigen (prokaryotic purified human SETD3 protein), dilute the purified monoclonal antibody according to the ratio in Table 2, and measure the A450 absorbance of each well, the results are shown in Table 3 and image 3 shown;
[0124] table 3
[0125] potency 1K 2K 4K 8K 16K 32K 64K 128K 256K Absorbance 0.580 0.579 0.480 0.441 0.340 0.230 0.163 0.109 0.077
[0126] From Table 3 and image 3 It can be seen that the antibody titer can reach 1:128000.
Embodiment 3
[0127] Embodiment 3, antibody specific detection
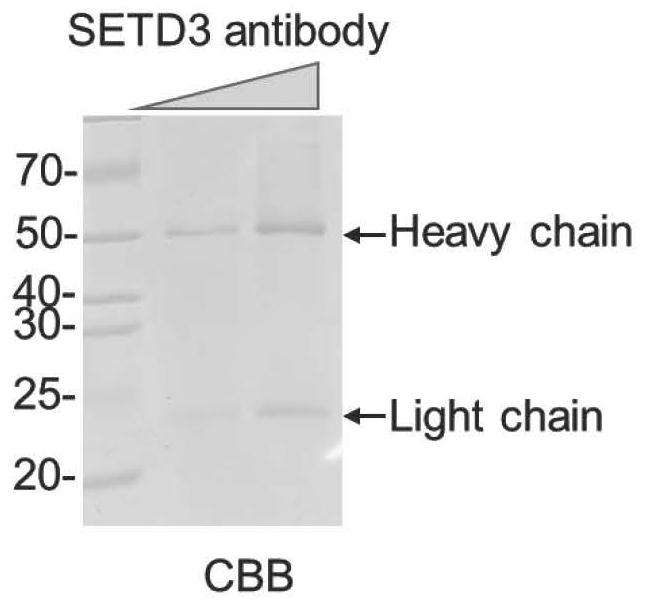
[0128] 1. Use in vitro purified SETD3 protein to detect antibody specificity.
[0129] Purify GST-tagged SETD3 protein or pure GST-tagged protein in vitro, and use Western Blot to test the recognition specificity of the purified antibody, such as Figure 4 As shown, the left picture shows the loading of the two proteins by Coomassie Brilliant Blue staining, and the right picture shows the specificity of the antibody detected by Western Blot experiment. The results show that the SETD31-H9 monoclonal antibody has a good recognition specificity for GST-SETD3 , did not recognize the equivalent amount of GST protein.
[0130] 2. Detection of antibody specificity at the cellular level
[0131] (1) Construct SETD3 knockdown or overexpression cell line in mammalian cell PC9 cell line, detect with 1-H9 antibody, such as Figure 5 shown. The method for constructing the SETD3 knockdown or overexpression cell line in the embodiment of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com