Antigen polypeptide recognized by lppz antibody and use thereof
An antigen polypeptide and antibody recognition technology, applied in the field of biomedicine, can solve the problems of reduced specificity, increased false positives, and high production costs, and achieves the effects of low cost, simple preparation and high detection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Preparation of Polypeptide
[0075] 1. Preparation of Peptide Library
[0076] The amino acid sequence of LppZ (Rv3006) was obtained from the protein public resource database uni-prot, and its sequence from N-terminal to C-terminal is as follows:
[0077] MWTTRLVRSGLAALCAAVLVSSGCARFNDAQSQPFTTEPELRPQPSSTPPPPPPLPPVPFPKECPAPGVMQGCLESTSGLIMGIDSKTALVAERITGAVEEISISAEPKVKTVIPVDPAGDGGLMDIVLSPTYSQDRLMYAYISTPTDNRVVRVADGDIPKDILTGIPKGAAGNTGALIFTSPTTLVVMTGDAGDPALAADPQSLAGKVLRIEQPTTIGQTPPTTALSGIGSGGGLCIDPVDGSLYVADRTPTADRLQRITKNSEVSTVWTWPDKPGVAGCAAMDGTVLVNLINTKLTVAVRLAPSTGAVTGEPDVVRKDTHAHAWALRMSPDGNVWGATVNKTAGDAEKLDDVVFPLFPQGGGFPRNNDDKT(SEQ ID NO:4)。
[0078] The full-length sequence of the LppZ protein was split into a group of 15 amino acid-length polypeptides, and 12 amino acid residues were sequentially overlapped between adjacent polypeptides to form the LppZ peptide library, with a total of 121 peptides.
[0079] 2. In situ synthesis of LppZ protein peptide library to...
Embodiment 2
[0081] Example 2 Screening for polypeptides related to tuberculosis (screening of antigenic epitopes)
[0082] 1. Hydration and Western Blotting of Peptide Chips
[0083] The polypeptide chip prepared in Example 1 (herein referred to as "polypeptide chip 1") was immersed in 40 ml of 100% ethanol and shaken on a shaker for 5 minutes. Then, the polypeptide chip was immersed in 40 ml of 75% ethanol and shaken on a shaker for 5 minutes. Next, immerse the peptide chip in 40ml of 50% ethanol and shake it on a shaker for 5 minutes. Next, add 150ml PBS and soak for 30 minutes. Finally, immerse the peptide chip in 40ml of 5% skim milk powder / PBS-T solution, and incubate at room temperature for 3 hours to block.
[0084] Dilute the serum samples involved in the following primary screening and identification experiments with 5% skimmed milk powder / PBS-T solution 1:1000 to prepare an immune reaction solution, put the peptide chip into the hybridization bag, and add the reaction solutio...
Embodiment 3
[0095] Example 3 ELISA test
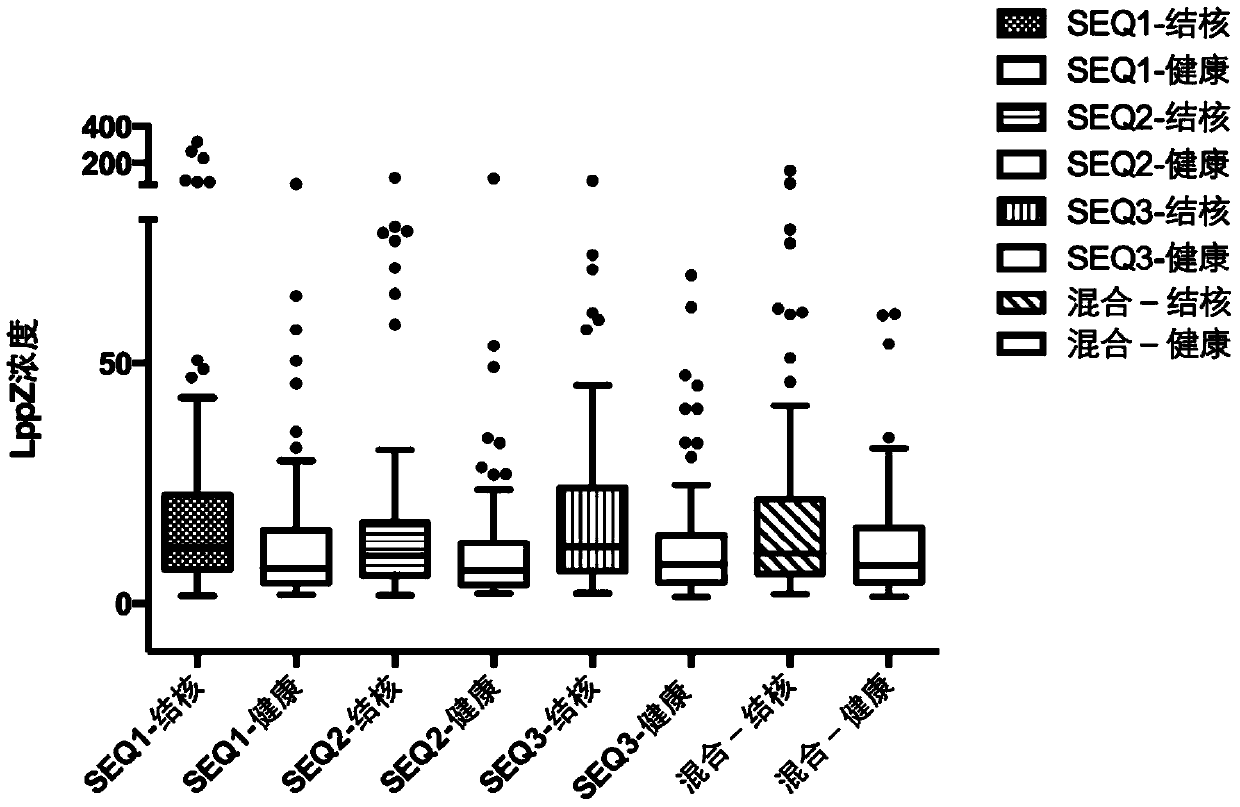
[0096] The three polypeptides (shown in Table 1) screened in Example 2 were used as antigens to coat 96-well plates, and the ELISA method was used to detect LppZ antibodies in the following samples: 82 tuberculosis patient sera and 38 healthy control sera.
[0097] The specific detection method is as follows:
[0098] Firstly, the antigenic polypeptide was synthesized according to the sequence of amino acid residues in the sequence listing using the polypeptide synthesizer MultiPep RS.
[0099] Next, use pH 9.6 carbonate buffer (NaCO 3 0.159g, NaHCO 3 0.293g, add water to 100ml, pH value is 9.6) dilute the peptide to 1μg / ml, add 100μl / well to the wells of a 96-well microplate, and seal the plate overnight at 4°C.
[0100] Next, discard the liquid in the well, 0.1% TBS-T washing buffer (the TBS-T solution containing 0.1% Tween-20 is prepared as NaCl 8.7g, Tris 1.21g, add deionized water to 1000ml, pH 7.5, Then add Tween-20 1ml) to wash the mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com