Application of combination of ACTR10 and CA125 in ovarian cancer detection and kit
A CA125, detection reagent technology, applied in the field of medical diagnosis, can solve problems such as insufficient diagnostic sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Differential detection of ACTR10 expression in stage I ovarian cancer patients and non-ovarian cancer individuals
[0076] Different detection methods of ACTR10 can detect the difference in the expression of stage I ovarian cancer patients and non-ovarian cancer individuals.
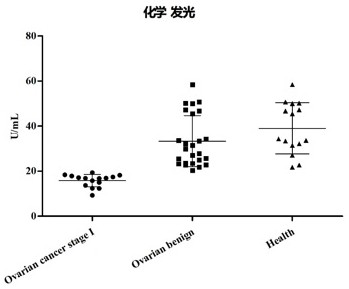
[0077] The concentration of ACTR10 was detected by chemiluminescent method, and the results are shown in figure 1 : The average value of patients with stage I ovarian cancer is 16.2U / ml (that is, 16.2U ACTR10 protein per ml of plasma), and the average value of patients with benign ovarian diseases is 33.3 U / ml. The p value of the two groups of statistical tests is less than 0.0001. The average value of healthy individuals is 39.01 U / ml, and the p-value of the statistical test between the stage I ovarian cancer patient group and the healthy individual group is less than 0.0001.
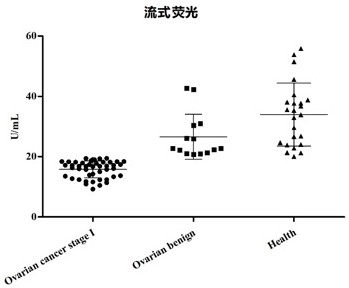
[0078] The concentration of ACTR10 was detected by flow fluorescence method, and the results are shown in figure 2 : ...
Embodiment 2
[0081] Expression detection of ACTR10 in different cancer patients
[0082] ACTR10 is specifically expressed in plasma vesicles of ovarian cancer patients. In the prior art solutions, the method for detecting several miRNAs in vesicles will affect the specificity of ovarian cancer diagnosis. The expression of the same miRNA is increased in a variety of tumors.
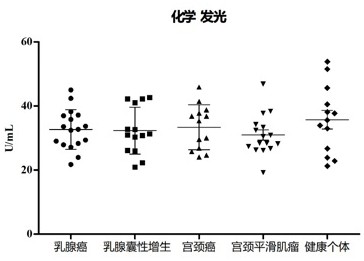
[0083] In this example, ACTR10 in different cancer patients was detected by chemiluminescence, and the results are shown in image 3 : ACTR10 was not significantly different in plasma vesicles of patients with other common tumors or benign diseases in women; in breast cancer, breast benign cystic hyperplasia, cervical cancer, cervical benign leiomyoma and corresponding healthy individuals, the There was no significant difference in concentration, and the average values of each group were: 32.65, 32.32, 33.37, 30.94, 35.73 U / ml.
Embodiment 3
[0085] The combination of ACTR10 and CA125 is used for auxiliary diagnosis of early ovarian cancer.
[0086] A total of 100 gynecological outpatients were enrolled. The inclusion criteria were CA125 in the range of 35U to 100U / ml, abdominal discomfort, and vaginal ultrasound showing ovarian space-occupying lesions, but laparoscopy had not yet been performed. Use the chemiluminescence platform to detect ACTR10 in the plasma vesicles of the above 100 women and substitute the value of CA125 into the auxiliary diagnosis model of ovarian cancer: OCS1=0.38×C CA125 -0.66×C ACTR10 , C CA125 Represents the concentration value of CA125 in the subject's plasma, C ACTR10 Represents the concentration of ACTR10 in the subject's plasma vesicles; judging method: if C CA125 ≥35 (ie CA125 concentration ≥35U / ml) and 4.7CA125 <35 and -11<OCS1<10.3, the subject is judged to be stage I ovarian cancer.
[0087] According to whether the 100 model results are in the interval of 4.7 to 27.8, the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com