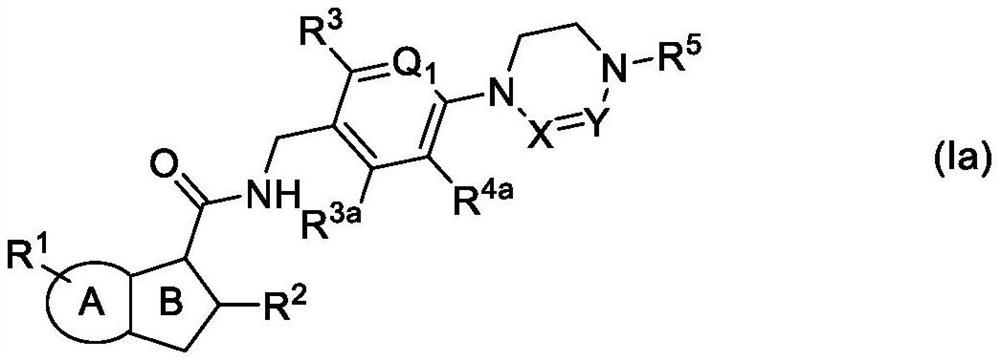
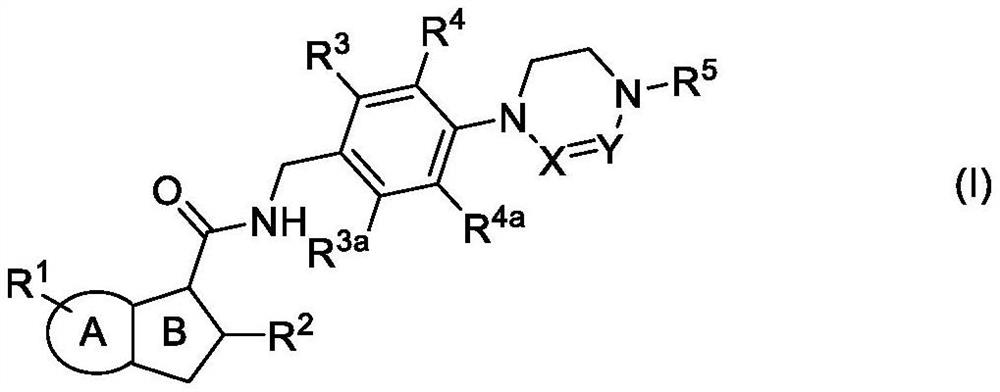
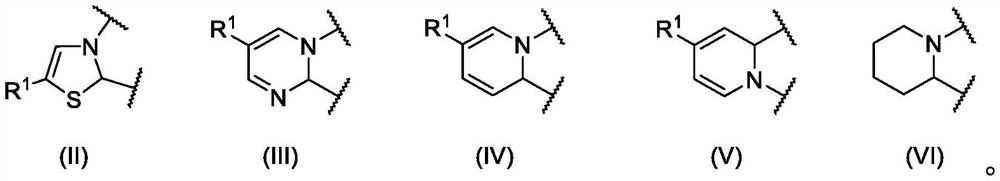
Antibacterial compounds
A compound and alkyl technology, applied in antibacterial drugs, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as morbidity, mortality and financial cost increase burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0243] 1. general information
[0244] melting point
[0245] Melting points were recorded using a differential scanning calorimeter DSC 1 (Mettler Toledo). Melting points were measured with a temperature gradient of 10°C / min from 25°C to 350°C. Values are peak values. Use this method unless otherwise stated.
[0246] An alternative is to use an open capillary on a Mettler Toledo MP50, denoted at "MT". Using this method, melting points are measured with a temperature gradient of 10°C / min. The highest temperature is 300°C. Melting point data was read from a digital display and checked from a video recording system.
[0247] 1 H NMR
[0248] Using an internal deuterium lock and equipped with an inverted double resonance with a z-gradient ( 1 H, 13C, SEI) probe and on a Bruker Avance DRX 400 spectrometer or Bruker Advance III 400 spectrometer operating at 400 MHz for protons and 100 MHz for carbon, and on a Bruker 5mm BBFO probe equipped with a z-gradient and on a Br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com