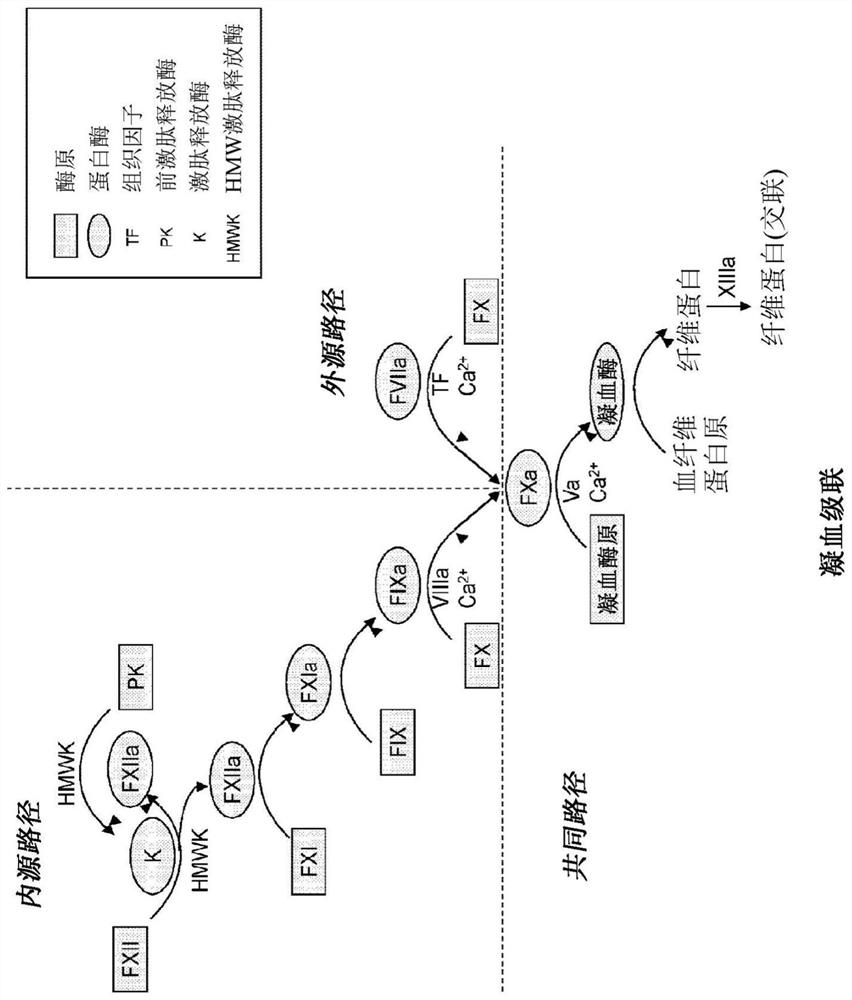
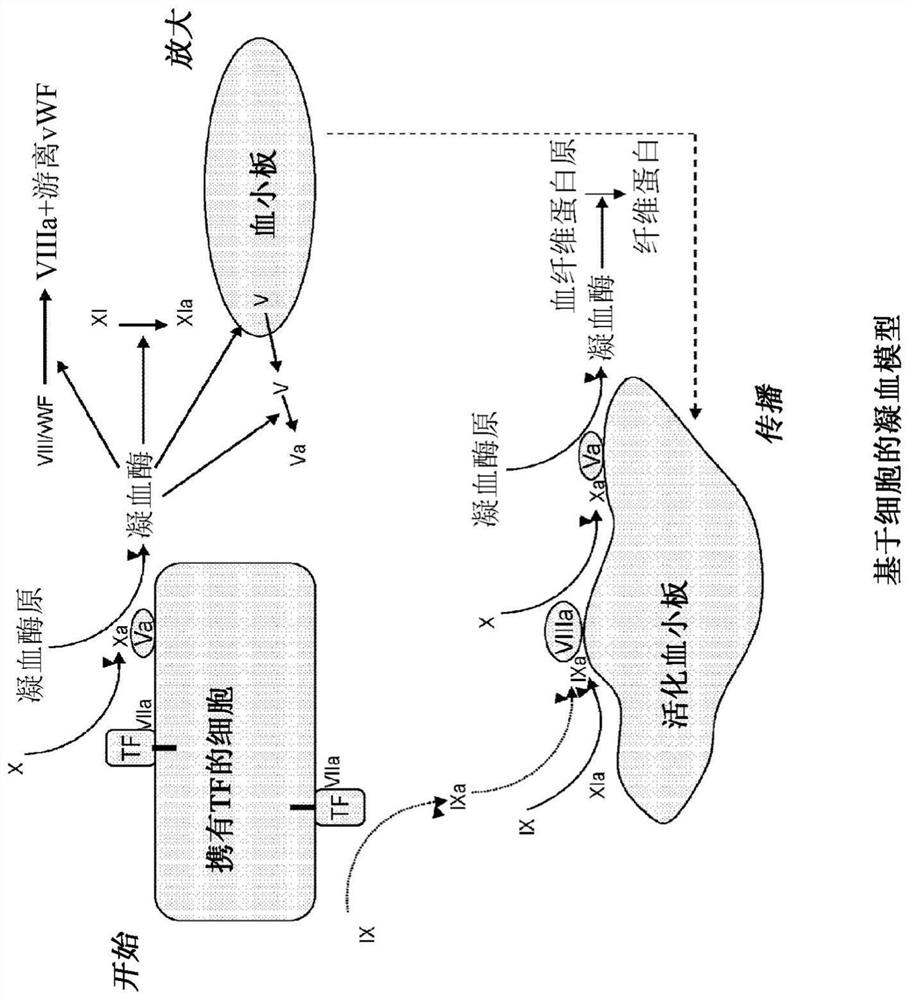
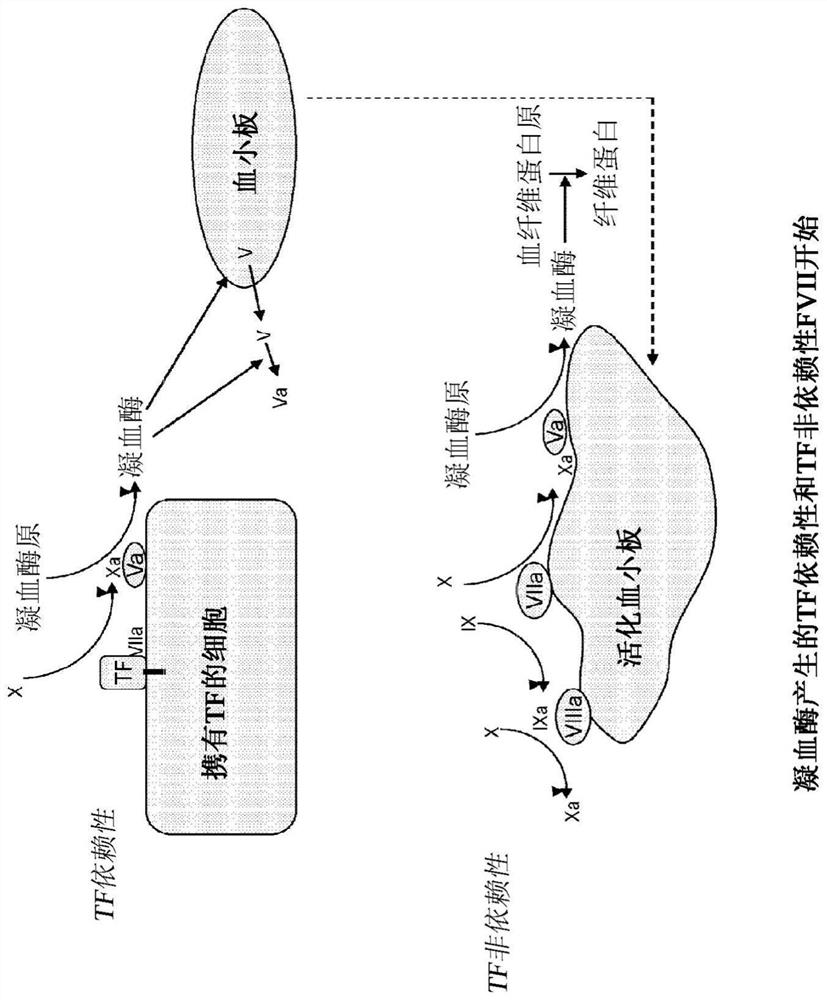
Modified factor VII polypeptides for subcutaneous administration and on-demand treatment
A technology for subcutaneous administration of factors, applied in the direction of anticoagulant factors immunoglobulin, peptides, peptidases, etc., can solve the problem of lack of treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-1
[0191] Embodiment 1-1. A method of treating a bleeding event in a subject comprising subcutaneously administering a dose of Modified Factor VIIa (FVIIa), wherein:
[0192] The modified FVIIa has greater activity or potency than FVIIa, FVIIa is unmodified and the amino acid sequence is set forth in SEQ ID NO: 3; and
[0193] A dose of modified FVIIa is administered subcutaneously within about 5 or 4 or 3 or 2 or 1 or less hours or minutes before and / or after a bleeding event to reduce or stop the amount of bleeding or to correct or cure the cause of the bleeding.
Embodiment I-2
[0194] Embodiment 1-2. The method of embodiment 1-1, wherein the subcutaneous dose of modified FVIIa is administered subcutaneously every 3-7, 2-5, 4-6 or 4-12 hours until bleeding ceases or causes Corrected, or any wound healed or persisted for 1 to 2, 3, 4 or 5 days.
Embodiment I-3
[0195] Embodiment 1-3. The method of embodiment 1-1 or 1-2, wherein the dose of modified FVIIa is per 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 hours.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap